Squamous-cell carcinoma of the skin
Authors:
Mikael Häggström; Authors of integrated Creative Commons article[1] [note 1]
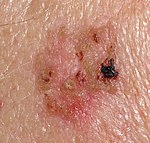
Squamous-cell carcinoma (SCC) of the skin may present as suspected malignant skin excisions.
Contents
- 1 Fixation
- 2 Gross processing
- 3 Microscopic evaluation
- 4 Re-excisions
- 5 Notes
- 6 Main page
- 7 References
- 8 Image sources
Fixation
Generally 10% neutral buffered formalin.
See also: General notes on fixation
Gross processing
If re-excision, see separate section at bottom.
Gross examination
Note:
- Color
- Well-defined or diffuse border
- Size
- Any elevation
Squamous cell carcinoma in situ (essentially synonymous with Bowen’s disease) often presents as an erythematous, well-demarcated, scaly patch or plaque, with a fairly irregular border. They occasionally present as dark skin focalities, especially when found in the genital region and the nails.[1]
Invasive SCC typically has ill-marginated, erythematous, scaling, and rough papules or patches.[1]
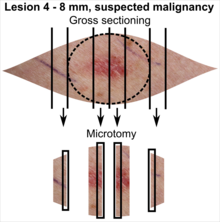
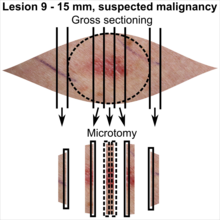
Tissue selection
| <4 mm | 4 - 8 mm | 9 - 15 mm |
|---|---|---|

|
|
|
In table above, each top image shows recommended lines for cutting out slices to be submitted for further processing. Bottom image shows which side of the slice that should be put to microtomy. Dashed lines here mean that either side could be used. Further information: Gross processing of skin excisions
Microscopic evaluation
Evaluation consists of:
- Determining whether it is a SCC rather than a differential diagnosis.
- Distinguishing a SCC in situ from an invasive SCC
- Radicality, and if radical, determine the least distance to a margin.
Characteristics

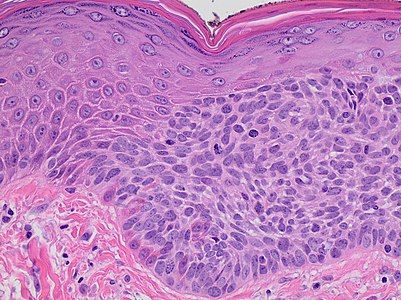
- Malignant keratinocytes demonstrating intense mitotic activity, pleomorphism, and greatly enlarged nuclei. They will also show a loss of maturity and polarity, giving the epidermis a disordered or “windblown” appearance.
In situ
In SCC in situ (Bowen’s disease) the epidermis will show:
- Atypia spanning the full thickness of the epidermis, being the main finding.[1]
- Hyperkeratosis and parakeratosis.[1]
- Marked acanthosis with elongation and thickening of the rete ridges. These changes will overly keratinocytic cells which are often highly atypical and may in fact have a more unusual appearance than invasive SCC.
- Typical squamous-cell carcinoma cells are large with abundant eosinophilic cytoplasm and large, often vesicular, nuclei.[3]
- Two types of multinucleated cells may be seen:[1]
- Multinucleated giant cells
- Dyskeratotic cells engulfed in the cytoplasm of keratinocytes.
- Occasionally, cells of the upper epidermis will undergo vacuolization.[1]
There may be a mild to moderate lymphohistiocytic infiltrate detected in the upper dermis.[1]
| Atypia spanning the full thickness of the epidermis is enough in this case for the diagnosis of SCC in situ. There is also a lymphohistiocytic infiltrate. |
Squamous cell-like skin proliferations: Differential diagnosis
Main differential diagnoses and their characteristics:[4]
Invasive squamous-cell carcinoma of the skin: Atypical and pleomorphic keratinocytes, involving the dermis and the sub-cutis with a potential metastatic spread.
Squamous-cell carcinoma in situ (Bowen’s disease): Atypical keratinocytes at every layer of epidermis.
Actinic keratosis: Atypical keratinocytes that do not span the full thickness of the epidermis (or, in Bowenoid variant, are less disordered with less nuclear atypia and crowding).
- Keratoacanthoma (2197016163).jpg
Keratoacanthoma: Symmetrical and circumscribed proliferation of keratinocytes, with central horn plug, with epidermis that extends over the tumor. It can be regarded as a highly differentiated SCC.
Adenosquamous carcinoma: Mixed glandular and squamous differentiation.
Verrucous squamous cell carcinoma[note 3]: Exophytic squamous proliferation with marked papillomatosis and low atypia and the presence of koilocyte-like changes. Found in head and neck locations, as well as in the genitalia and sole of the foot.
Inverted follicular keratosis:[note 4]: Sharply circumscribed endophytic verrucous proliferation with prominent squamous features.
Seborrheic keratosis: Acanthosis, absence of atypia, pseudo-horn cysts, in inflamed lesions, mitoses may be present.
A melanoma may have relatively plentiful eosinophilic cytoplasm, and be seemingly continuous with the squamous epithelium (at left in image), thus resembling a squamous cell carcinoma. However, the nesting of cells at right in the image is more characteristic of a melanoma.
In contrast to actinic keratosis, the basal epidermal layer in SCC in situ is frequently spared, and will show little to no visible atypia. Additionally, SCC in situ will almost always involve both the interfollicular and adjacent follicular epithelium and adnexal structures.[1]
Overlap of squamous-cell and basal-cell carcinoma
Basal-cell carcinoma is generally distinguishable by for example relatively less cytoplasm, palisading, cleft formations and absence of horn cyst formation.
Yet, a high prevalence means a relatively high incidence of borderline cases. In such cases, look particularly at the surface and attempt to classify as either of the following:
Basaloid squamous-cell carcinoma, in this case showing a biplastic pattern with basaloid elements associated with both conventional dysplastic squamous surface (arrow heads) and conventional squamous cell carcinoma (arrow).[5]
In unclear cases, the most useful immunohistochemistry marker appears to be MOC-31, which essentially always stains metatypical basal-cell carcinomas but not basaloid squamous-cell carcinomas.[6] UEA-1 appears to be the second most useful marker, staining almost all basaloid squamous-cell carcinomas but only a few metatypical basal-cell carcinomas.[6]
Clinical clues
- Biopsy from sun exposed area (including the face, neck, dorsal hands, and forearms, upper chest, back, and scalp).[1]
- Generally middle-aged or older individuals.[1]
In situ versus invasive
- In situ (Bowen's disease)
- Intact basement membrane.
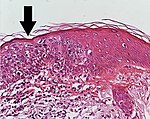
High magnification, demonstrating an intact basement membrane.[1]
- Invasive SCC
Invasive SCC is defined by dermal infiltration.
Superficially invasive squamous cell carcinoma (SCCSI). These lesions often do not show the marked pleomorphism and atypical nuclei of SCC in situ, but demonstrate early keratinocyte invasion of the dermis.[1]
High magnification demonstrates the pleomorphism of the invading keratinocytes.[1]
This infiltrate can be somewhat difficult to detect in the early stages of invasion: however, additional indicators such as full thickness epidermal atypia and the involvement of hair follicles can be used to facilitate the diagnosis. Later stages of invasion are characterized by the formation of nests of atypical tumor cells in the dermis, often with a corresponding inflammatory infiltrate.[1]
Radicality
Determine whether the distances between atypical cells are more or less than 1 mm from the deep and radial edges. If less than 1 mm, quantify the distance.[7]
Degree of differentiation
This is applicable to invasive SCC.
Well-differentiated (and yet invasive) SCC, showing prominent keratinization and may form “pearllike” structures where dermal nests of keratinocytes attempt to mature in a layered fashion. Well-differentiated SCC has slightly enlarged, hyperchromatic nuclei with abundant amounts of cytoplasm. Intercellular bridges will frequently be visible.[1]
Moderately differentiated lesions of invasive SCC show much less organization and maturation with significantly less keratin formation.[1]
Poorly differentiated, where attempts at keratinization are often no longer evident. This is a clear-cell squamous cell carcinoma. The dysplastic cells here infiltrate in cords through the dermis. Poorly differentiated SCC has greatly enlarged, pleomorphic nuclei demonstrating a high degree of atypia and frequent mitoses.[1]
Perineural or vascular invasion
In SCC, look for any perineural invasion,[note 5] and at least a quick glance for any vascular invasion.
Perineural invasion: the arrow indicates a large peripheral nerve that has been surrounded by tumor cells.[1]
Vascular invasion: the arrow indicates a small cluster of atypical squamous cells in a small vessel.[1]
Perineural invasion is defined as tumor in close proximity to nerve and involving at least 33% of its circumference or tumor cells within any of the three layers of the nerve sheath (epineurium, perineurium and endoneurium).[8] First look along the border of the tumor, followed by surrounding tissue, and if still not found, look through the rest of the tumor area as well.[1]
Staging
The AJCC, 8th Ed., does not include any staging system for skin SCC, except for tumors of the vulva.[9]
Optionally: Grading
Multiple variables can be combined to classify a SCC as low or high grade:
| Low-Grade SCC[1] | High-Grade SCC[1] |
|---|---|
|
|
Further work-up
In vulvar squamous cell carcinoma, generally perform p16 immunohistochemistry, which is considered a surrogate marker for oncogenic HPV infection.[10]
Microscopy report
On this resource, the following formatting is used for comprehensiveness:
- Minimal depth
- (Moderate depth)
- ((Comprehensive))
Components of the report:
- Diagnosis of squamous-cell carcinoma
- Whether it is in situ or invasive. If invasive:
- Degree of differentiation.
- (High or low grade.)
- Even absence of perineural invasion[note 5]
- ((Even absence of or vascular invasion.))
- Radicality, mainly into either of the following:
- >1 mm (as per Radicality above): "Clear margins" (or: "Clear margins at over __ mm")((or the exact distance thereof)).))
- <1 mm but not continuous with edge: "Close margins at __ mm at (location). [[Locations are mainly the deep edge, or the (superior/inferior/medial/lateral) radial edge.]]."[7] Numbers are generally given at an exactness of 0.1 mm.
- Continuous with margin: "Not radically excised at (location)."
- Staging is only applicable for the head and neck (lip, ear, face, scalp and neck - see staging at Medscape) and vulva (see staging at cancer.net).
((You may also add a synoptic report (see examples):))
Examples
Squamous cell carcinoma in situ:
| ((Skin excision with squamous epithelium with))(central parakeratosis. The epidermis is thickened and exhibits disturbed stratification. )All cell layers show atypical epithelial cells with polymorphic and partially hyperchromatic nuclei. The basement membrane is intact. Clear margins. ((There is elastosis and inflammatory cells in the dermis.)) |
Invasive squamous cell carcinoma:
| (Skin, right breast, excision:) Invasive keratinizing squamous cell carcinoma, well differentiated, measuring 1.7 cm in greatest dimension. Surgical margins are negative for carcinoma. (Negative for lymphovascular and perineural invasion.) ((Solar elastosis.)) |
((Example synoptic report:))
- Procedure: Skin excision.
- Tumor site: Scalp
- Tumor laterality: Right
- Tumor focality: Unifocal
- Tumor size: 1.6 x 1.4 cm
- Tumor depth of invasion: 0.3 cm
- Histologic type: Squamous cell carcinoma
- Histologic grade: Moderately differentiated
- Specimen margins: Uninvolved by invasive tumor.
- Lymphovascular invasion: Not identified
- Perineural invasion: Not identified
- Regional lymph nodes: No lymph nodes submitted or found.
- Pathologic Stage Classification (pTNM, AJCC 8th Edition):
- Primary Tumor: pT1
- Regional Lymph Nodes: pNX: Regional lymph nodes cannot be assessed
- Additional pathologic findings: Actinic keratosis
Example microscopic description of invasive squamous cell carcinoma:
- Squamous epithelium, with central ulceration, surrounded by hyperkeratosis. In this area in the dermis there are infiltrative nests of epithelioid cells with nuclear pleomorphism and <sparse / moderate / abundant> keratin formation.
See also: General notes on reporting
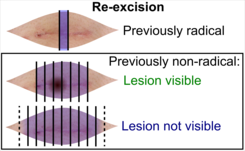
Re-excisions
Gross processing
((Submit the entire specimen, or)) depending on radicality of previous excision:
- Previously radical (including thin margins): Submit at least one central section across the surgical scar.[11]
- Previously non-radical:
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
- ↑ The excision example shows a superficial basal cell carcinoma.
- ↑ - Buschke–Löwenstein tumor is an alternative name for verrucous squamous cell carcinoma in the ano-genital region.
- Carcinoma cuniculatum is a characteristic form of verrucous squamous cell carcinoma on the sole. - ↑ Inverted follicular keratosis is generally thought to be a rare variant of seborrheic keratosis, but this position is not universally accepted.
- Karadag, AyseSerap; Ozlu, Emin; Uzuncakmak, TugbaKevser; Akdeniz, Necmettin; Cobanoglu, Bengu; Oman, Berkant (2016). "Inverted follicular keratosis successfully treated with imiquimod ". Indian Dermatology Online Journal 7 (3): 177. doi:. ISSN 2229-5178. - ↑ 5.0 5.1 Presence or absence of perineural invasion in squamous-cell carcinoma affects whether adjuvant radiotherapy will be used.
Main page
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 Yanofsky, Valerie R.; Mercer, Stephen E.; Phelps, Robert G. (2011). "Histopathological Variants of Cutaneous Squamous Cell Carcinoma: A Review
". Journal of Skin Cancer 2011: 1–13. doi:. ISSN 2090-2905..
-"This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited." - ↑ There are many variants for the processing of skin excisions. These examples use aspects from the following sources:
- . Handläggning av hudprover – provtagningsanvisningar, utskärningsprinciper och snittning (Handling of skin samples - sampling instructions, cutting principles and incision. Swedish Society of Pathology.
- For number of slices and coverage of lesions, depending on size. - Monica Dahlgren, Janne Malina, Anna Måsbäck, Otto Ljungberg. Stora utskärningen. KVAST (Swedish Society of Pathology). Retrieved on 2019-09-26.
- For slices towards the pointy ends to determine radicality, which can be parallel to the slices through the lesions (shown), or as longitudinal slices that go through each pointy end. - . Dermatopathology Grossing Guidelines. University of California, Los Angeles. Retrieved on 2019-10-23.
- For microtomy of the most central side at the lesion - "The principles of mohs micrographic surgery for cutaneous neoplasia
- With a "standard histologic examination" that, in addition to the lesion, only includes one section from each side along the longest diameter of the specimen.
- It also shows an example of circular coverage, with equal coverage distance in all four directions.
- The entire specimen may be submitted if the risk of malignancy is high. - . Handläggning av hudprover – provtagningsanvisningar, utskärningsprinciper och snittning (Handling of skin samples - sampling instructions, cutting principles and incision. Swedish Society of Pathology.
- ↑ Dr Nicholas Turnbull, A/Prof Patrick Emanual (2014-05-03). Squamous cell carcinoma pathology. DermNetz.
- ↑ Initially copied from: Paolino, Giovanni; Donati, Michele; Didona, Dario; Mercuri, Santo; Cantisani, Carmen (2017). "Histology of Non-Melanoma Skin Cancers: An Update
". Biomedicines 5 (4): 71. doi:. ISSN 2227-9059.
"This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/)." - ↑ El-Mofty, SK. (2014). "Histopathologic risk factors in oral and oropharyngeal squamous cell carcinoma variants: An update with special reference to HPV-related carcinomas
". Medicina Oral Patología Oral y Cirugia Bucal: e377–e385. doi:. ISSN 16986946.
License: CC BY 2.5 - ↑ 6.0 6.1 Webb, David V.; Mentrikoski, Mark J.; Verduin, Lindsey; Brill, Louis B.; Wick, Mark R. (2015). "Basal cell carcinoma vs basaloid squamous cell carcinoma of the skin: an immunohistochemical reappraisal ". Annals of Diagnostic Pathology 19 (2): 70–75. doi:. ISSN 10929134.
- ↑ 7.0 7.1 1 mm as cutoff for close margin: Brodie M Elliott, Benjamin R Douglass, Daniel McConnell, Blair Johnson, Christopher Harmston (2018-12-14). New Zealand Medical Journal.
- ↑ Strowd, Roy (2021). Neuro-oncology for the clinical neurologist . Philadelphia, PA: Elsevier. ISBN 978-0-323-69494-0. OCLC 1220993756.
- ↑ Amin, Mahul (2017). AJCC cancer staging manual
(8 ed.). Switzerland: Springer. ISBN 978-3-319-40617-6. OCLC 961218414.
- For access, see the Secrets chapter of Patholines.
- Copyright note: The AJCC, 8th Ed. is published by a company in Switzerland, and the tables presented therein are Public Domain because they consist of tabular information without literary or artistic innovation, and therefore do not fulfil the inclusion criterion of the Swiss Copyright Act (CopA) which applies to "literary and artistic intellectual creations with individual character" (see Federal Act on Copyright and Related Rights (Copyright Act, CopA) of 9 October 1992 (Status as of 1 January 2022)). - ↑ Anjelica Hodgson, M.D., Carlos Parra-Herran, M.D.. p16. Pathology Outlines. Last author update: 1 July 2017. Last staff update: 20 July 2022
- ↑ 11.0 11.1 Katarzyna Lundmark. Handläggning av hudprover – provtagningsanvisningar, utskärningsprinciper och snittning (Handling of skin samples - sampling instructions, cutting principles and incision. Swedish Society of Pathology.
- ↑ Pathology Department at NU Hospital Group, Sweden, 2019-2020.
Image sources