Template:Fixation, gross processing and evaluation
Contents
- 1 Fixation
- 2 Gross processing
- 3 Evaluation
Fixation
Immersion
Within an hour after removal from the body,[1] tissue samples should generally be placed in vessels with enough fixative to allow them to lie freely in the solution.[2] The standard fixation fluid is generally 10% neutral buffered formalin, which is roughly equivalent to 4% formaldehyde.[3] The ratio of tissue:formalin should be 1:5[4] to 1:10[5].[5]
Duration
The duration depends on tissue thickness, where formalin will penetrate and fix the tissue at ~1 mm/hour.[6]
When not to use formalin
The main exceptions to using formalin are mainly:
- Intraoperative consultation.
- Suspected crystals, such as a tophus or other specimen suspicious for gout versus pseudogout. These should be sent in alcohol or dry, since formalin will dissolve the crystals.
- Suspected lymphoproliferative disorders, such as lymph nodes (or other lymphoid aggregates) with a suspicion of lymphoma, where samples are generally put in a special solution for flow cytometry.
- Need for genetic testing, such as some cases of products of conception.
- Cytology specimens, which are preferably sent fresh (such as in red top tubes) to be processed within a few hours. If processing may be after a few hours, put tubes on ice, or add 50% alcohol.[7]
- Need for microbiology evaluation, mainly bacterial culture. For potentially infectious workups, check with the microbiology lab if they have the tissue they need before putting the specimen in formalin.
- Need for immunofluorescence, such as immune complex-mediated disease, where specific preservation will give better test sensitivity.[8]
If you don't know, and if you cannot soon get in touch with anyone who can guide you, specimens can generally be stored in a fridge in the meantime, even overnight if it is late (but make sure to follow-up as soon as possible in the morning). Until then, don't put the specimen in formalin and don't freeze the specimen.
Gross processing
Following are general notes on selection and trimming in pathology.
Priority
|
1. More invasive intraoperative consultations |
For prioritizing when you have more than one thing ongoing at the same time, the list at right can be used.
Fresh specimens
The initial processing of fresh specimens is also termed triaging.
- For intraoperative consults including frozen sectioning, see separate article on Emergent pathology.
When triaging a specimen, the measurements are most important as these may change after fixation. Other descriptions can generally be made after the specimen is fixed.
- See also separate article on fixation, including what specimens should not immediately be put in formalin (mostly tophus, lymph nodes and products of conception).
Before cutting
- Confirm that the patient identity on the specimen container matches the identity that will be applied to the gross description and cassettes. {{If the referral or requisition form is available, confirm the patient identity on that one as well.}}
- (Check for any discrepancy between the specimen description on the container and on the referral or requisition form, such as left versus right.)
- Don't omit any piece in the container, such as ones hidden in wraps.
- Generally measure in 3 dimensions, or in volume, but the greatest dimension is generally enough for specimens less than 0.4 cm.
- Generally weigh entire organs, after having any attached tissue trimmed away if feasible.
- (Note the color of the sample, even when unremarkable, but do not linger on deciding it.)[note 1]
- Generally, use inking for resection margins where tumor radicality is important. Further information: inking
- (On fatty or greasy surfaces, apply vinegar to emulsify and remove the fat, dry the specimen and then ink. Otherwise, vinegar can be used either before or after inking to "dry" it.)
- (Preferably photograph or make a drawing where slices have been taken.)[9]
- Remove any surgical stitches from samples before microtomy.
- (At least for larger samples, consider looking for medical imaging or biopsy reports in order to better guide the process.)[10]
- Fix bone in formalin prior to decalcification. Use reminders so not to forget bone that is decalcifying.
- Plan in which order to cut different parts so as to be able to take all relevant sections. Generally sample relevant surgical margins and smaller parts first, as these may be harder to find or even be fragmented later on.
Cutting
- When cutting with the longer knives, try to cut in one stroke - do not use like a saw (continuous back and forth)
- Generally, strive to make slices perpendicular to visible interfaces of relevant tissues.
- Generelly dissect and inspect the entire specimen, while keeping relevant parts intact enough for presentation to seniors and/or maintaining orientation.
- Trim tissues for microscopy examination to a thickness of maximum 3-4 mm.[note 2]
Perpendicular versus en face sections
Two major types of sections in gross processing are perpendicular and en face sections:
- Perpendicular sections allow for measurement of the distance between a lesion and the surgical margin.
- En face means that the section is tangential to the region of interest (such as a lesion) of a specimen. It does not in itself specify whether subsequent microtomy of the slice should be performed on the peripheral or proximal surface of the slice (the peripheral surface of an en face section is closer to being the true margin, whereas the proximal surface generally displays more area and therefore generally has greater sensitivity in showing pathology, also compared to perpendicular sections).
- A shaved section is a superficial en face slice that contains the entire surface of the segment.
Tissue selection
When sampling sections to submit for microscopic examination, whenever you sample from something that looks abnormal, generally try to also sample from the same type of tissue that looks normal.[note 3]
Biopsy wraps, bags and sponges
Put the following types of specimens in bags:
- Tiny specimens that need to be poured out from their containers.
- Bloody specimens such as endometrial curettages or products of conception. For products of conception, chorionic villi may otherwise contaminate other specimens. Bloody specimens may stick to wraps, so generally avoid that situation.
- Friable tissue such as urinary bladder biopsies.
Put the following types of specimens in bags, wraps or sponges:
- Other tiny specimens
- ((Any small piece of tissue where there is no leftover specimen to retake sections, since tissues occasionally get lost from cassettes, and the absence of a wrap, sponge or bag in the cassette of such cases points towards a mistake made at gross processing.))
Specimens must be fixed enough to be put on sponges.
H&E staining urgency
(Even in departments where other staff are primarily responsible for determining the urgency of H&E staining of each specimen, still double-check that it is correct if you can, such as by cassette color.) A major indication for rushing cases through H&E staining is a high risk of cancer, especially where immunohistochemistry staining will likely be performed, and the decision and types of staining will be determined by the standard H&E stain. Tissues that are generally rushed are:
- Brain biopsy.
- Lung biopsy.
- Breast needle biopsy.
- Biopsy from known tumor tissue.
- Suspected malignant lymph nodes, including lymphoma. However, these are generally not urgent when submitted together with a tumor, except mainly for the following (which are generally urgent):
- Pelvic sentinel lymph nodes
- Sentinel lymph nodes from known invasive lobular carcinoma (but not invasive ductal carcinoma)
In both these cases, the cases are rushed so that immunohistochemistry can be performed if a metastasis is not readily detected on standard H&E slides, so that it is available by the time the rest of the slides are out. Immunohistochemistry in these cases can detect micrometastases that are not readily visible on H&E stain, but are evident on cytokeratin AE1/AE3. However, if the lab stains such cases regardless of whether H&E stain shows a metastasis or not, then they do not need to be rushed.
Marking cassettes
Use only hard pencil (or specially purchased histology markers), as marks made with pens, Sharpie markers, or scientific freezer-safe markers can get dissolved in tissue processing.[11]
Evaluation
First, confirm that the identity of the sample evaluated is the same as that of the requisition form and/or other medical history.
Microscopy settings
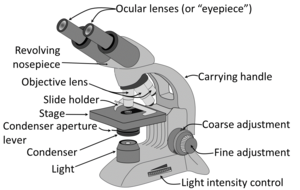
Generally the condenser is placed in its highest position or just slightly lower. At low magnification objectives (mainly 4x and 10x objectives), the opening of the condenser (or iris) diaphragm should be wide open. This corresponds to turning away or "lowering" the condenser on microscopes where the condenser apparatus can be turned to the side (and is shown as "without condenser" in images below). For high-dry (40x) and oil-immersion objectives (100x), the diaphragm should be closed slowly while looking at a sharply focused section until the level of illumination is just slightly reduced, in order to attain optimal contrast and resolution (and corresponds to "with condenser" in images below).[12]
Low magnification has a greater span of focus compared to high magnification, so it is normal to need to focus if you're increasing magnification. If there's a constant visual artifact, even after you've cleaned the eye piece and objective lenses with lens tissue, try raising or lowering the condenser if you can, and the artifact may disappear out of focus.
Priority
Whenever you have more than one case, generally have an initial look at the requisition form and/or in the microscope at the most likely relevant area of each case, and prioritize the case(s) that likely need to be done first. Indications to prioritize case(s) are mainly:
- Clinical management of the patient highly benefits from a fast diagnosis.
- Being marked as a rush case.
- There's a significant probability that it will require further workup such as immunohistochemistry, especially when there is an impending deadline for ordering it.
- The optimal person to ask for advice may not be available later. Further information: Consultation
As part of initial triaging, also determine what tasks can be 'delegated to juniors or pathologist assistants.
For a pile of many cases of relatively low risk of a need to prioritize any one over another, such as gastrointestinal biopsies, a very quick glance at the forms and/or a naked eye look at the glass slides is generally sufficient.
Main steps
- Preferably, look up past medical history of the patient, mainly past cancers that could possibly appear in the current specimen.
- Try to pick up glass slides without touching the tissue area, as fingerprints may interfere with the evaluation.
- Look at each microscopy slide by plain eye, to plan the microscopy scanning so as to not miss peripheral fragments.
- Have a systematic direction of scanning through microscopy slides, such as from top left to bottom right as seen in the microscope. When the microscope makes what you see two-way mirrored, the starting position is with the objective pointing at the bottom right of the glass slide. You may center on findings on interest and evaluate them at higher magnification, and then resume the scanning
This scanning technique above makes sure that you don't miss any area, and also minimizes redundant scanning. The width or clock position that you scan in each field of view is not exact, as you may scan up to the entire width in cases where you think relevant findings will clearly present themselves even if only partially showing in the periphery of your view.
- Look in particular for whatever is requested or suspected on the requisition form or equivalent. For each type of condition, initially you will generally focus relatively more on high magnification features with high specificity, but you should still have a habit of looking at low magnification as well to get an idea of its pattern. In time, you will increasingly correlate diseases and conditions with their overall low magnification patterns - patterns that may require 1000 words to describe and thus cannot conveniently be part of written criteria, but will nevertheless allow you to make quicker and more accurate diagnoses, or at least clues thereof.
- When you find something suspicious, it is helpful at least in the beginning to evaluate them systematically by a low-to-high magnification approach (example below showing a basal-cell carcinoma):
- Complete the scanning even if you encounter a finding, as there may be additional findings as well. It is usually helpful to Google multiple images of the normal histology of the location you are looking at, and compare those to what you see, and focus on whatever features may deviate from the normal.
General patterns
Following are major patterns that often help in making a diagnosis.
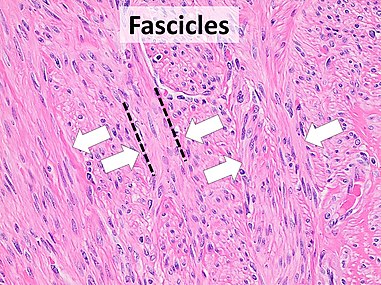
Architectural patterns
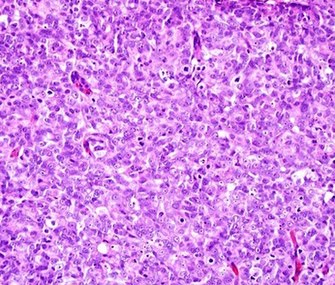
Cellular patterns
Nuclear patterns
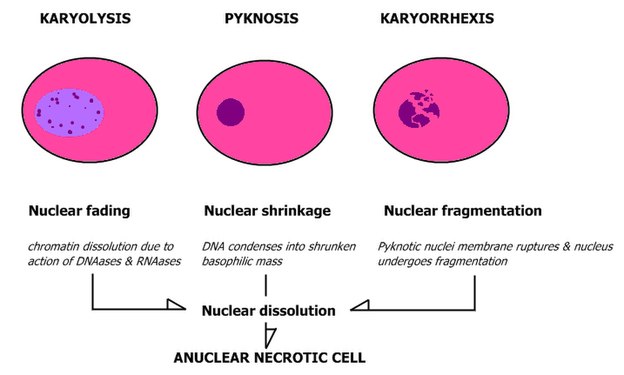
When feasible, classify nuclei as follows:
Pleomorphic when having different sizes and shapes. This often correlates with an increased nucleus to cytoplasm ratio. These features generally favor malignancy in the evaluation of suspected malignancies.
- Patterns of nuclear disintegration
Other patterns
Inflammation
Marking slides
...Then slowly approach the slide while making sure that the tip remains in the location you want to mark. Be careful not to obscure findings of interest with the mark. If the slide is likely to undergo whole slide imaging, make the mark on the back and not on the coverslip (as it reduces the risk of erroneous focusing of the cameras).
Measuring distances
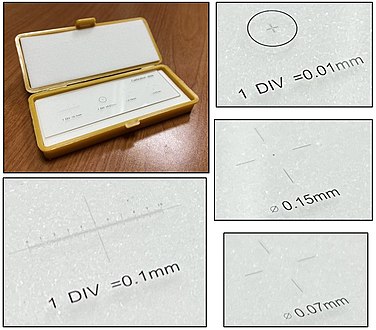
There are also eye pieces that show a ruler in the field of view, but make sure you match it with the correct objective and other settings to make the measurement valid. A calibration slide is more robust because of such sources of error.
Counts per mm2
There are multiple situations where a finding will be quantified in terms of amount per mm2. To make such calculations, you need to know the size of the area you see in the microscope. It is usually possible to look up what theoretically would be the area, but the most reliable way of knowing is to use a calibration slide to measure the diameter of your view. The area is then calculated as:
- Area in mm2 ≈ (diameter in mm2) x 0.79
On the imaged example, each square is 0.05 mm wide, and each line represents 0.01 mm, making the diameter of the field of view 0.55 mm in this case. Thus, you can calculate the area in mm2 by Googling:
- 0.55 x 0.55 x 0.79
- (equals approximately 0.24 mm2).
Generally count at least 10 fields of a high power view (or more if specifically instructed). You can keep the count in your head by repeating the total count and the order of the area you are looking at, such as:
- "Zero (instances) of one (field of view)"
- "One of two (as you see one instance as you've moved to the second field of view)"
- "Two of two" etc.
Subsequently, the count per mm2 is calculated as follows:
| Count per mm2 = | Total count |
| Number of fields x Area per field (in mm2) |
For example, if you reached "15 of 10", and the area of your field is 0.2 mm2, the count per mm2 can be calculated by Googling:
- 15/10/0.2
- = 7.5
Sometimes "high power field" (HPF) is used for area, but it has a substantially different area for different microscopes, for example:
| Microscope type | Area per HPF |
|---|---|
|
0.096 mm2 [13] |
| AO with 10x eyepiece | 0.12 mm2 [13] |
| Nikon Eclipse E400 with 10x eyepiece and 40x objective | 0.25 mm2 |
| Leitz Ortholux | 0.27 mm2 [13] |
| Leitz Diaplan | 0.31 mm2 [13] |
When your instructions are to count a specific number of HPFs, one HPF can be assumed to be 0.2 mm2.[14] If the view area in your microscope significantly differs from this area, calculate how many views you need to count as:
| Views = HPFs required x | 0.2 |
| Your microscope area (in mm2) |
For example, if your instruction is to count 10 HPFs and each view in your microscope shows 0.096 mm2, you should count in this many views:
| 10 x | 0.2 | ≈ 21 |
| 0.096 |
Subsequently, if your microscope area is significantly different from 0.2 mm2 and you need to state your result in terms of count/HPF, use:
| Count/HPF = Average count in your view x | 0.2 |
| Area of your view in mm2 |
For example, if you have counted an average of 10 cells (or other object of interest) in each of your views, and the area of your view is 0.096 mm2, then your count/HPF is:
| 10 x | 0.2 | ≈ 21 |
| 0.096 |
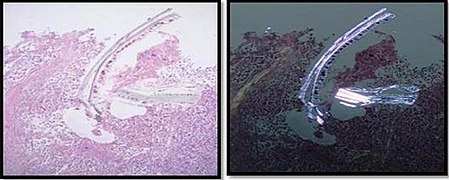
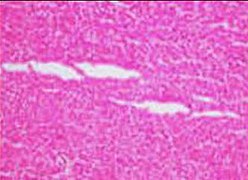
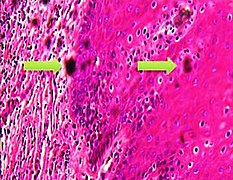
Artifacts
In microscopy, an artifact is an apparent structural detail that is caused by the processing of the specimen and is thus not a legitimate feature of the specimen. Major artifacts to account for include:
Differential diagnoses of artifacts are mainly:
- Foreign bodies. In contrast to contamination, these conform more naturally to the surrounding tissue.
- Organisms, to be particularly considered when there are multiple objects of the same size.
Order recuts from the same paraffin-embedded tissue if artifacts significantly impairs your diagnostic evaluation of the glass slide. However, artifacts caused by gross processing may affect recuts as well.
Micrography and telepathology
Unless you have more specific equipment for taking microscopic images and showing cases to remote colleagues, you can perform these tasks as follows:
- With a stationary computer, you can connect to a microscopy camera. For telepathology, you can start a videoconferencing session with your senior, then share the screen while showing a micrograph, or the live view so that you can move around.
- With a mobile phone:
- You can send micrographs to your senior, but it is technically difficult to keep the focus while moving the glass slide.
Consideration
Consider the adequacy of the specimen. Have a somewhat lower threshold to make a report of "inadequate" or "insufficient" if it is easy to resample from the patient, such as remaining diagnostic tissue at a superficial location.
First, generally suspect the common conditions for the location at hand. A less common variant of a common condition is often still more likely than a rare condition, so generally call the latter only if it really fits the picture.
Whenever you consider a certain diagnosis, also consider whether it is one step better or worse. For example, when you consider a non-invasive but high-grade dysplasia, also consider both low-grade dysplasia and invasiveness. </noinclude>
|
Further reading: |
Notes
- ↑ The color of gross specimens generally has very limited clinical significance.
- ↑ Thicker slices may not become adequately fixated in formalin.
- ↑ Normal sections from the same tissue helps identifying what is histologically abnormal in the grossly abnormal tissue, versus normal individual variations.
Main page
References
- ↑ . Breast pathology grossing guidelines. UCLA Health. Retrieved on 2021-09-09.
- ↑ Katarzyna Lundmark, Krynitz, Ismini Vassilaki, Lena Mölne, Annika Ternesten Bratel. Handläggning av hudprover – provtagningsanvisningar, utskärningsprinciper och snittning (Handling of skin samples - Instructions for sampling, cutting and incision. KVAST (Swedish Society of Pathology). Retrieved on 2019-09-09.
- ↑ . Paraformaldehyde, Formadehyde and Formalin. Duke University. Retrieved on 2019-12-17.
- ↑ . Fixation of Tissues. Approval Date: August 2016, August 2020. Review Date: August 2024|website=Royal College of Pathologists of Australia
- ↑ 5.0 5.1 Buesa RJ, Peshkov MV (2012). "How much formalin is enough to fix tissues? ". Ann Diagn Pathol 16 (3): 202-9. doi:. PMID 22483550. Archived from the original. .
- ↑ . How to Submit Tissues for Embedding. University of Pittsburgh, Starzl Transplantation Institute. Revised 04/19/21
- ↑ . How to send fluid and make good cytology slides. Tufts University.
- ↑ Mubarak M, Kazi Javed I, Kulsoom U, Ishaque M (2012). "Detection of immunoglobulins and complement components in formalin fixed and paraffin embedded renal biopsy material by immunoflourescence technique. ". J Nephropathol 1 (2): 91-100. doi:. PMID 24475396. PMC: 3886135. Archived from the original. .
- ↑ Monika Roychowdhury. Grossing (histologic sampling) of breast lesions. Pathologyoutlines.com. Topic Completed: 1 August 2012. Revised: 19 September 2019
- ↑ . Gross Pathology Manual By The University of Chicago Department of Pathology. Updated 2-14-19 NAC.
- ↑ . Histopathology Services. UNC School of Medicine. Retrieved on 2021-11-15.
- ↑ Patrice F Spitalnik. Histology Laboratory Manual, Vagelos College of Physicians & Surgeons Columbia University. Retrieved on 2021-09-20.
- ↑ 13.0 13.1 13.2 13.3 . Infiltrating Ductal Carcinoma of the Breast (Carcinoma of No Special Type). Stanford University School of Medicine. Retrieved on 2019-10-02.
- ↑ Klimstra, David S.; Modlin, Irvin R.; Coppola, Domenico; Lloyd, Ricardo V.; Suster, Saul (2010). "The Pathologic Classification of Neuroendocrine Tumors ". Pancreas 39 (6): 707–712. doi:. ISSN 0885-3177.
- ↑ 15.0 15.1 15.2 Taqi, SyedAhmed; Sami, SyedAbdus; Sami, LateefBegum; Zaki, SyedAhmed (2018). "A review of artifacts in histopathology ". Journal of Oral and Maxillofacial Pathology 22 (2): 279. doi:. ISSN 0973-029X.
Image sources
</noinclude>