Template:Lungs and lymph nodes
Contents
Lungs
Author:
Mikael Häggström [note 1]
Basic microscopic screening
- For screening of lung autopsies, see Lung autopsy
Mainly look for carcinoma. Further information: Lung tumor
If granulomas are seen, generally stain for acid-fast bacteria and fungi.
Common findings
Active searching for them is not mandatory.
Anthracosis (interstitial black material).
Respiratory epithelial shedding in a small bronchus. If present, look for vascular leakage, mucus hypersecretion and/or widespread airway narrowing, together indicating asthma.[1]
Other pertinent findings
Pulmonary aspergillosis, seen as acutely branching septated hyphae.[2]
Pulmonary mucormycosis, seen as non-septated and broad-branching, and sometimes branching at right angles (black arrow).[3]
For fungi not conforming to the two main forms above, a general pathologist may attempt to get input by readily available expertise locally, but if it cannot be readily speciated, then it's generally acceptable to simply report as fungi present.
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
Main page
References
- ↑ Madea, B (2014). Handbook of forensic medicine . Hoboken, N.J: Wiley-Blackwell. ISBN 978-1-118-57062-3. OCLC 872114659.
- ↑ Error on call to Template:cite web: Parameters url and title must be specified. . Wellcome Collection. Retrieved on 2024-02-21. License: CC0 1.0 Universal
- ↑ Lee JH, Hyun JS, Kang DY, Lee HJ, Park SG (2016). "Rare complication of bronchoesophageal fistula due to pulmonary mucormycosis after induction chemotherapy for acute myeloid leukemia: a case report.
". J Med Case Rep 10: 195. doi:. PMID 27423701. PMC: 4947348. Archived from the original. .
- "This article is distributed under the terms of the Creative Commons Attribution 4.0 International License"
Image sources
Lung tumor
Gross processing
As per presentation above.
Microscopic evaluation
Medical imaging provides a major clue as to whether a lung tumor is benign or malignant, where lesions smaller than 2 cm are likely to be benign, whereas lesions larger than 2 cm are malignant (that is, lung cancer) in 85% of cases.[1]
Benign tumors
Subsequently distribution of benign tumors and lung cancers, respectively, are as follows:[1]
Benign lung tumors:
- Hamartomas - 76%
- Benign fibrous mesothelioma/solitary fibrous tumor (SFT) - 12.3%
- Inflammatory pseudotumor (IPT) - 5.4%
- Lipoma - 1.5%
- Leiomyoma - 1.5%
- Other - 3.3%
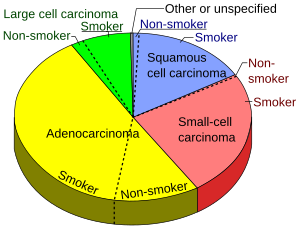
Lung cancers
Lung adenocarcinoma, with lepidic pattern shown, wherein tumors cells cover alveolar walls.
Lung adenocarcinoma, with solid pattern.
Squamous-cell carcinoma (SCC) of the lung. Typical squamous-cell carcinoma cells are large with abundant eosinophilic cytoplasm and large, often vesicular, nuclei.[3]
Small-cell carcinoma, with typical findings.[4]

Whereas large cell carcinoma is more often histologically distinct, adenocarcinoma and SCC may look alike. In such cases, an immunohistochemistry panel of TTF1, CK5/6, and p63 can be used to distinguish the two.[6][7]
Further workup
For primary lung non-small cell carcinoma (NSCLC) stages IB - IV (such as being more than 3 cm in size), generally perform full next generation sequencing panel (DNA and RNA) with PDL-1 immunostaining. For an advanced stage NSCLC that is not a candidate for biopsy or re-biopsy, a viable alternative is “liquid biopsy” on peripheral blood for circulating tumor DNA.[8]
Notes
Main page
References
- ↑ 1.0 1.1 Alain C. Borczuk (2008). "Benign Tumors and Tumorlike Conditions of the Lung ". Archives of Pathology & Laboratory Medicine 132 (7). Archived from the original. .
- ↑ Kuroki, Masaomi; Nakata, Hiroshi; Masuda, Toshifumi; Hashiguchi, Norihisa; Tamura, Shozo; Nabeshima, Kazuki; Matsuzaki, Yasunori; Onitsuka, Toshio (2002). "Minute Pulmonary Meningothelial-like Nodules: High-Resolution Computed Tomography and Pathologic Correlations ". Journal of Thoracic Imaging 17 (3): 227–229. doi:. ISSN 0883-5993.
- ↑ Dr Nicholas Turnbull, A/Prof Patrick Emanual (2014-05-03). Squamous cell carcinoma pathology. DermNetz.
- ↑ Image by Mikael Häggström, MD. Source for findings: Caroline I.M. Underwood, M.D., Carolyn Glass, M.D., Ph.D.. Lung - Small cell carcinoma. Pathology Outlines. Last author update: 20 September 2022}}
- ↑ Image by Mikael Häggström, MD. Source for significance: Bejarano PA, Mousavi F (2003). "Incidence and significance of cytoplasmic thyroid transcription factor-1 immunoreactivity. ". Arch Pathol Lab Med 127 (2): 193-5. doi:. PMID 12562233. Archived from the original. .
- ↑ Inamura K (2018). "Update on Immunohistochemistry for the Diagnosis of Lung Cancer. ". Cancers (Basel) 10 (3). doi:. PMID 29538329. PMC: 5876647. Archived from the original. .
- ↑ Affandi KA, Tizen NMS, Mustangin M, Zin RRMRM (2018). "p40 Immunohistochemistry Is an Excellent Marker in Primary Lung Squamous Cell Carcinoma. ". J Pathol Transl Med 52 (5): 283-289. doi:. PMID 30235512. PMC: 6166010. Archived from the original. .
- ↑ . National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines) - Non-Small Cell Lung Cancer. Version 3.2024. Section: Principles of molecular and biomarker analysis (2024-03-12).
Image sources
- ↑ Image(s) by: Mikael Häggström, M.D. Public Domain
- Author info
- Reusing images
Lung wedge resection and lobectomy
Author:
Mikael Häggström [note 1]
Comprehensiveness
On this resource, the following formatting is used for comprehensiveness:
- Minimal depth
- (Moderate depth)
- ((Comprehensive))
- Other legend
<< Decision needed between alternatives separated by / signs >>
{{Common findings / In case of findings}}
[[Comments]]
Link to another page
Intraoperative consultation
Perform:
- Tumor microscopy on frozen sectioning if there is intermediate risk of cancer (if frozen section does not show cancer, the surgeon may not need to perform lymph node dissection)
- Margin assessment: A gross distance from tumor to the parenchymal is generally sufficient, unless a suspected malignancy is close enough to confer a significant risk of extension to the margin, in which case the closest parenchymal margin should be frozen en face[note 2]. For lobectomies, generally perform frozen section on the bronchial and vascular margin en face[note 2].
Grossing
Perform the following:[1]
- Measure the specimen in 3 dimensions.
- (Weigh lobectomies.).
- Describe pleural surface, including color, and any presence of granularity, adhesions, retraction, or tumor.
- Palpate for any tumors.
- Ink the surgical margin and cut it away just below any sutures or staples. If the margin is substantially stapled (and their removal would be either too tissue-damaging or otherwise inconvenient), ink and use another section of the tissue underneath it for frozen sectioning.
- In intraoperative consultations use a section that is presumably closest to a tumor for frozen sectioning, with the tissue en face[note 2], for radicality. This is generally enough to report intraoperatively to the surgeon, unless otherwise requested.
- ((Sample the entire surgical margin for standard processing.))
- Cut open the bronchi of the specimen with a pair of scissors, as far as they can fit within the lumina. Attempt to cut so as to be able to take a section that includes both any tumor and nearest bronchus. Palpate for tumors intermittently. Describe the cut surface, including color and consistency, and any focal lesions.
- Turn the specimen to the side with least cuts so far, and serially section it. Palpate for tumors intermittently.
|
- For any found lung tumor:
- Measure tumor size as a maximum diameter (or 3 dimensions)
- Determine location: Which lobe if applicable, and if it is peripheral, central or hilar.
- Margin length to pleura and hilum/surgical margin.
- Any involvement of major bronchi or blood vessels.
- Describe any lymph nodes, including location, range of sizes and appearance of cut surface.
Tissue selection
- 1 from bronchial and vascular margins, en face[note 2], if present, ((differentially inked))
- 1 from nearest parenchymal margin, en face
- Sections of any tumor
- Any other focal change
- 1 from random non-neoplastic lung tissue
Gross report
| ((A. Labeled - ___. The specimen is received fresh for intraoperative consultation and consists of)) of a right upper lobe of lung which measures __ x __ x __ cm (and weighs __ g). The specimen includes a bronchial stump measuring __ cm in length and __ cm in diameter, which grossly appears unremarkable. The pleural surface is mottled tan-pink {{and slightly puckered on the __ aspect}}. There is a staple line representing the parenchymal margin measuring __ cm in length. The stapled margin is inked black. (On opening the bronchial tree, the mucosa is tan and smooth and the lumens are patent. The blood vessels are opened to reveal no blood clot or tumor.) {{Cut section show an irregular, gray-tan, rubbery firm mass measuring __ x __ x __ cm. The tumor is located __ cm from the bronchial and vascular margin and __ cm from the nearest surgical margin. The tumor abuts smaller bronchi and vessels.}} The remaining parenchyma is pink and spongy. (No lymph nodes are identified in the peribronchial region.) (Representative sections are submitted for microscopic examination in __ cassettes.) |
See also: General notes on gross processing
Microscopic evaluation
Look mainly for carcinoma. Further information: Lung tumor
Microscopy report
Lung synoptic reports contain information (number and station) on all lymph nodes received per accession. For example, if Parts A-D are mediastinal nodes (8 in total) and Part E is a lobectomy containing 2 additional peribronchial nodes, the synoptic report for Part E should document all 10 nodes, for example:
A. Lymph node, station 1:
Negative for carcinoma. (0/1, 2 etc)
B. Lymph node, station 2:
Negative for carcinoma. (0/1, 2 etc)
C. Lobectomy, RLL: Adenocarcinoma
- Size:
- Histologic type
- Margins
See also: General notes on reporting
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
- ↑ 2.0 2.1 2.2 2.3 En face means that the section is tangential to the region of interest (such as a lesion) of a specimen. Further information: Gross_processing#Cutting
Main page
References
- ↑ Partially using the following procedure:. Pulmonary pathology grossing guidelines. Retrieved on 2021-03-17.
Image sources
Lymph nodes
Author:
Mikael Häggström [note 1]
Comprehensiveness
On this resource, the following formatting is used for comprehensiveness:
- Minimal depth
- (Moderate depth)
- ((Comprehensive))
Gross processing
If suspected lymphoma, before putting tissue in formalin, ensure that tissue is preserved in appropriate media for any special tests (usually flow cytometry). Further information: Lymphoma
In samples with tumors, slice through all included fat while palpating and looking for lymph nodes, and submit all that are found.
For lymph nodes taken for potential breast cancer metastasis, find out and report the procurement time and the time when put in formalin.[note 2]
Gross procedure
- Measure the dimensions. For a lymph node with minimal surrounding fatty tissue, measure the greatest dimension (or 3 dimensions). For specimens with substantial amount of fatty tissue, measure the specimen in 3 dimensions, and measure the greatest dimension seen for individual lymph nodes therein after serial sectioning.
- Find as many lymph nodes as you can in a specimen. Good locations to start include the presumed lymphatic drainage directions from a tumor, as well as when following the lymphatic directions from the vascular margins of a specimen. Serially section fatty tissue into slices that are thin enough to be palpated for small ovoid resistances. If you still have trouble finding enough lymph nodes, put fatty tissue in a vinegar and acetic acid solution made for the purpose of turning lymph nodes pale/white as well as making them more firm for palpation. Colon tumors are sometimes tattooed during endoscopy, and in such cases the tatoo ink often stains lymph nodes as well.
- Section lymph nodes if needed. Lymph nodes less than 5 mm may be submitted whole, while larger lymph nodes may be sectioned at 2-3 mm intervals.[1]
- Generally do not submit multiple sectioned lymph nodes in the same cassette, to allow exact counting of the number of involved lymph nodes on microscopy. If you will nevertheless submit multiple bisected lymph nodes in the same cassette, ink each lymph node differently.
- If suspected lymphoma, such as an enlarged lymph node without any adjacent tumor or another almost certain cause, make a touch prep. Also, take a small fresh sample for flow cytometry:
- For flow cytometry, aim for a tissue size of approximately 5 mm3. Put it in specific flow cytometry preservative medium (such as RPMI), and ensure it gets to the flow cytometry lab. If it is after normal hours and there is no one to ask to find such medium, you can put the specimen in normal sterile saline (enough to cover the tissue) in a fridge (2-8°C) until the next morning.[2] If you receive multiple lymph nodes for flow cytometry, still only sample one (unless the referral asks for separate flow cytometry studies, or there is a given history of one lymph node having high uptake and another having low uptake on PET scanning).
Definition of an enlarged lymph node
- By size, where lymphadenopathy in adults is often defined as a short axis of one or more lymph nodes is greater than 10mm.[3][4] However, there is regional variation as detailed in this table:
| Generally | 10 mm[3][4] |
| Inguinal | 10[5] – 20 mm[6] |
| Pelvis | 10 mm for ovoid lymph nodes, 8 mm for rounded[5] |
| Neck | |
|---|---|
| Generally (non-retropharyngeal) | 10 mm[5][7] |
| Jugulodigastric lymph nodes | 11mm[5] or 15 mm[7] |
| Retropharyngeal | 8 mm[7]
|
| Mediastinum | |
| Mediastinum, generally | 10 mm[5] |
| Superior mediastinum and high paratracheal | 7mm[8] |
| Low paratracheal and subcarinal | 11 mm[8] |
| Upper abdominal | |
| Retrocrural space | 6 mm[9] |
| Paracardiac | 8 mm[9] |
| Gastrohepatic ligament | 8 mm[9] |
| Upper paraaortic region | 9 mm[9] |
| Portacaval space | 10 mm[9] |
| Porta hepatis | 7 mm[9] |
| Lower paraaortic region | 11 mm[9] |
Lymphadenopathy of the axillary lymph nodes can be defined as solid nodes measuring more than 15 mm without fatty hilum.[10] Axillary lymph nodes may be normal up to 30 mm if consisting largely of fat.[10]
In children, a short axis of 8 mm can be used.[11] However, inguinal lymph nodes of up to 15 mm and cervical lymph nodes of up to 20 mm are generally normal in children up to age 8–12.[12]
Lymphadenopathy of more than 1.5 cm - 2 cm increases the risk of cancer or granulomatous disease as the cause rather than only inflammation or infection.[13]
Urgency
The processing of lymph nodes is preferably rushed when the H&E stain will determine whether immunohistochemistry will be performed, especially when a lymph node is submitted together with a separate specimen that may be solved without immunostains. This rushing allows you to have the immunostained slides by a similar time as the rest of the case.[14] Examples of cases that are preferably rushed for such reasons include those that may be stained by CK AE1/AE3 in order to visualize otherwise occult lymph node involvement if you don't see any involvement on the H&E stain, mainly in cases when one or more sentinel lymph nodes are submitted together with any of the following:
- A breast biopsy or excision of a suspected or previously confirmed invasive lobular carcinoma (but not necessarily invasive carcinoma with lobular features)
- A uterus specimen of a suspected or previously confirmed endometrial cancer.
Rushing is not necessary for non-sentinel lymph nodes.
Gross report
- Individual lymph node, example
| ((A. Labeled - ___. The specimen is received in formalin and consists of)) __ fragment(s) of soft pink-tan tissue, measuring __ cm in greatest dimension (or __ x __ x __(. (Representative sections are submitted for microscopic examination in __ cassettes.) |
- Multiple lymph nodes
| ((A. Labeled - ___. The specimen is received fresh and consists of)) 2 irregular fragments of yellow-tan fatty and fibrous soft tissue measuring __ and ___ cm in greatest dimension. Within the adipose tissue are multiple tan-brown lymph nodes measuring up to __ cm in greatest dimension. The cut surfaces display no gross lesions. The lymph nodes are entirely submitted for microscopic examination (in 10 cassettes). KEY TO SECTIONS:
|
- Additional information
- If potential breast cancer metastasis: The specimen was procured at __ AM/PM on (date), 2020. The specimen was placed in formalin at __ AM/PM on (date), 2020.
- If lymphoma workup: A touch prep is made, and a minor part of the specimen is submitted for flow cytometry. The remainder of the specimen is submitted for microscopic examination in one cassette.
Microscopic examination
Defining a lymph node
For counting lymph nodes, each should have a discernible capsule around lymphoid cells. Also count larger free-standing lymphoid aggregates. However, the definition of what constitutes a lymph node is largely subjective.[15] Also strive to keep a consistency with the gross description. In addition, any cancer involvement is in itself a relative indication of being a lymph node.
General screening
Look for:
- Whatever pathology is indicated by the referral, or findings in other submitted specimens.
- Enlargement, as preferably measured during grossing, but can possibly be made on the microscopy slide. If present, see separate section below.
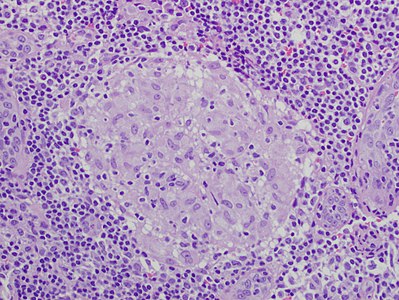
Metastases: generally first look around the edges with intermediate magnification, and low mag in the middle, since cancer metastases usually occur at edges (as in this case). For suspected urothelial cancers, however, look closely throughout the node, as they have a tendency to show up anywhere in lymph nodes.
Lymph node metastasis from a neuroendocrine tumor of the midgut. Metastates generally look similar to its primary tumor.
Microscopy of enlarged lymph nodes
Look at any other slides for the same case first, in order to find any pathology that may be reflected in in the lymph nodes as well, mainly cancer metastasis or reactive lymph nodes from inflammation.
Look primarily at the overall architecture, with main findings being:
Follicular hyperplasia: Indicates mainly follicular hyperplasia of a reactive lymph node (pictured) or follicular lymphoma. Further information: Follicular hyperplasia
Dilated sinuses. The most cellular expansion is sinus histiocytosis (pictured). If it appears as such, look for a signet ring appearance, which may be a signet ring carcinoma or melanoma. If unsure, use immunostains for CD68, cytokeratin, S100 and mucin.[16]
- Paracortical hyperplasia: Paracortical hyperplasia of a reactive lymph node shows expansion of paracortical areas by a mixed infiltrate, often having a mottled appearance, and it usually has a concomitant reactive follicular hyperplasia.[17] A T-cell lymphoma should be suspected if there is obliteration or marked diminution of the B-cell cortical region, or highly irregular or hyperchromatic nuclei.[17]
- Unspecific hyperplasia: An unspecific pattern of lymph node enlargement, without atypical cells, in the lymphatic drainage direction from an inflamed area, may simply be diagnosed as "benign reactive lymph node".
Workup of cancerous lymph nodes
If cancer is detected in a lymph node:
- Attempt to specify a specific cancer diagnosis'. If the patient has a known carcinoma or sarcoma etc, it is generally enough to confirm that it is consistent with a metastasis thereof.
- Measure the size of involvement.
- Look for extranodal extension.
Reporting
A non-involved lymph node in a patient with cancer can be reported for example as:
| Sentinel lymph node #1, left axilla, (excision): One benign lymph node((, negative for malignancy (0/1))). |
Cancerous lymph nodes with patients with known consistent cancer primary can be reported as metastatic,, such as:
| Sentinel lymph node #2, left axilla, (excision): Macrometastatic carcinoma involving one of one (1/1) lymph node. Metastatic carcinoma measures 0.4 cm in greatest dimension. (Negative for extranodal extension). |
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
- ↑ The duration that a specimen has been without formalin affects mainly the reliability of estreogen and progesteron receptor testing:
- Pekmezci, Melike; Szpaderska, Anna; Osipo, Clodia; Erşahin, Çağatay (2012). "The Effect of Cold Ischemia Time and/or Formalin Fixation on Estrogen Receptor, Progesterone Receptor, and Human Epidermal Growth Factor Receptor-2 Results in Breast Carcinoma ". Pathology Research International 2012: 1–7. doi:. ISSN 2090-8091.
Main page
References
- ↑ . Protocol for the Examination of Biopsy Specimens From Patients With Melanoma of the Skin. College of American Pathologists. Version: Melanoma Biopsy 4.1.0.0 Protocol Posting Date: August 2019
- ↑ . Specimen Information and Requirements for Flow Cytometry Testing. Lifelabs. Doc #8218 Ver: 7.0 Current Issued: 13-Apr-2018
- ↑ 3.0 3.1 Ganeshalingam, Skandadas; Koh, Dow-Mu (2009). "Nodal staging ". Cancer Imaging 9 (1): 104–111. doi:. ISSN 1470-7330. PMID 20080453.
- ↑ 4.0 4.1 Schmidt Júnior, Aurelino Fernandes; Rodrigues, Olavo Ribeiro; Matheus, Roberto Storte; Kim, Jorge Du Ub; Jatene, Fábio Biscegli (2007). "Distribuição, tamanho e número dos linfonodos mediastinais: definições por meio de estudo anatômico ". Jornal Brasileiro de Pneumologia 33 (2): 134–140. doi:. ISSN 1806-3713. PMID 17724531.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 "Current concepts in lymph node imaging ". Journal of Nuclear Medicine 45 (9): 1509–18. September 2004. PMID 15347718.
- ↑ . Assessment of lymphadenopathy. BMJ Best Practice. Retrieved on 2017-03-04. Last updated: Last updated: Feb 16, 2017
- ↑ 7.0 7.1 7.2 Page 432 in: Luca Saba (2016). Image Principles, Neck, and the Brain . CRC Press. ISBN 9781482216202.
- ↑ 8.0 8.1 Sharma, Amita; Fidias, Panos; Hayman, L. Anne; Loomis, Susanne L.; Taber, Katherine H.; Aquino, Suzanne L. (2004). "Patterns of Lymphadenopathy in Thoracic Malignancies ". RadioGraphics 24 (2): 419–434. doi:. ISSN 0271-5333. PMID 15026591. Archived from the original. .
- ↑ 9.0 9.1 9.2 9.3 9.4 9.5 9.6 Dorfman, R E; Alpern, M B; Gross, B H; Sandler, M A (1991). "Upper abdominal lymph nodes: criteria for normal size determined with CT. ". Radiology 180 (2): 319–322. doi:. ISSN 0033-8419. PMID 2068292.
- ↑ 10.0 10.1 Page 559 in: Wolfgang Dähnert (2011). Radiology Review Manual . Lippincott Williams & Wilkins. ISBN 9781609139438.
- ↑ Page 942 in: Richard M. Gore, Marc S. Levine (2010). High Yield Imaging Gastrointestinal HIGH YIELD in Radiology . Elsevier Health Sciences. ISBN 9781455711444.
- ↑ Laurence Knott. Generalised Lymphadenopathy. Patient UK. Retrieved on 2017-03-04. Last checked: 24 March 2014
- ↑ "Lymphadenopathy and malignancy ". American Family Physician 66 (11): 2103–10. December 2002. PMID 12484692.
- ↑ Chandler IP, Oommen R, Lawson CW (2003). "Invasive lobular carcinoma and cytokeratin immunohistochemistry: an audit. ". J Clin Pathol 56 (3): 240. doi:. PMID 12610108. PMC: 1769908. Archived from the original. .
- ↑ Parkash V, Bifulco C, Feinn R, Concato J, Jain D (2010). "To count and how to count, that is the question: interobserver and intraobserver variability among pathologists in lymph node counting. ". Am J Clin Pathol 134 (1): 42-9. doi:. PMID 20551265. Archived from the original. .
- ↑ Egan, Caoimhe; Jaffe, Elaine S. (2018). "Non-neoplastic histiocytic and dendritic cell disorders in lymph nodes ". Seminars in Diagnostic Pathology 35 (1): 20–33. doi:. ISSN 07402570.
- ↑ 17.0 17.1 Weiss, Lawrence M; O'Malley, Dennis (2013). "Benign lymphadenopathies ". Modern Pathology 26 (S1): S88–S96. doi:. ISSN 0893-3952.
Image sources