(Optionally: A. Container is labeled - __. The specimen is received in formalin and consists of ) 1 fragment(s) of pink-tan tissue with a vaguely recognizable mucosal surface. The tissue measures __ cm. (The surgical margin is inked black. The specimen is bisected and entirely submitted for microscopic examination in one cassette.)
Microscopic evaluation
Anatomic/Histologic location
Describe mucosa as squamous (or ectocervical), endocervical (generally mucinous and glandular) or transformation zone mucosa.
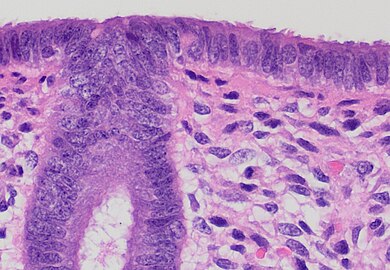
Squamous (or ectocervical) mucosa at left and endocervical (mucinous) mucosa at right, in an example with abrupt transition.
Transformation zone mucosa, consisting of a mix of stratified squamous epithelium and mucinous glands, in an example with gradual transition.
Endocervical mucosa, with mucinous columnar epithelium and mucinous glands. H&E stain
However, the endocervical mucosa may appear columnar and non-mucinous.
A cervical biopsy may contain nabothian cysts, which are single or multiple cysts that contain mucin, lined by a single layer of columnar, cuboidal to flat cells with variable amounts of mucinous cytoplasm and small, basal, round to oval nuclei with fine chromatin, without conspicuous nucleoli or mitotic activity.[1]
The anatomic level of the transformation zone varies:[2]
Type 1: Completely ectocervical (common under hormonal influence).
Type 2: Endocervical component but fully visible (common before puberty).
Type 3: Endocervical component, not fully visible (common after menopause).
If you see fragments of non-mucinous epithelium and glands, it is likely endometrial, so evaluate it like an endometrial curetting.
Pathologies
edit
Look for cervical dysplasia. It is mainly seen as nuclei with hyperchromasia, coarse chromatin and irregular contours.[3]
Spectrum from normal to high grade SIL.[4]
Further information: Cervical dysplasia
edit
Other common findings:
Acute cervicitis, having a largely neutrophilic intra-epithelial infiltrate.
Endocervical polyp: With endocervical epithelium and glands (mucinous columnar linings), edematous stroma and clear congestion. H&E stain.[5]
Example report
Endocervix, curettings:
Fragments of squamous and endocervical glandular epithelium without significant histopathologic changes.
Negative for dysplasia.
|
Notes
- ↑ 1.0 1.1 For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
Main page
References
- ↑ Gulisa Turashvili, M.D., Ph.D.. Cervix Benign / nonneoplastic epithelial lesions. Nabothian cysts.. Pathology Outlines. Last author update: 1 February 2021. Last staff update: 4 April 2022}}
- ↑ International Federation for Cervical Pathology and Colposcopy (IFCPC) classification. References:
-. Transformation zone (TZ) and cervical excision types. Royal College of Pathologists of Australasia.
- Jordan, J.; Arbyn, M.; Martin-Hirsch, P.; Schenck, U.; Baldauf, J-J.; Da Silva, D.; Anttila, A.; Nieminen, P.; et al. (2008). "European guidelines for quality assurance in cervical cancer screening: recommendations for clinical management of abnormal cervical cytology, part 1
". Cytopathology 19 (6): 342–354. doi:10.1111/j.1365-2303.2008.00623.x. ISSN 0956-5507. PMID 19040546.
- ↑ Khaled J. Alkhateeb, M.B.B.S., Ziyan T. Salih, M.D.. HSIL / CIN II / CIN III. PathologyOutlines. Topic Completed: 29 March 2021. Minor changes: 9 February 2022
- ↑ Source image by Ed Uthman from Houston, TX, USA. Creative Commons Attribution 2.0 Generic (CC BY 2.0) license
- ↑ Anissa Ben Amor.. Cervical Ectropion. StatPearls, National Center for Biotechnology Information. Last Update: November 14, 2021.
- This book is distributed under the terms of the Creative Commons Attribution 4.0 International License
Image sources
Cervical cone
Unless otherwise specified, the primary focus is any cervical neoplasia.
Fixation
Generally 10% neutral buffered formalin.
See also: General notes on fixation
Gross processing
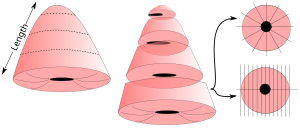
- Measure length, as well as the transverse and sagittal diameter of the ectocervical surface.[1]
- Optionally, weight the sample.[1]
- Note the symmetry of the sample, and the position of the cervical canal.[1]
- Note whether the circumference is complete. If not, and the directions are indicated on the cone, determine the approximate position of the defect.[1]
- Cones excised by knife should be inked on the excision surfaces. Those excised by laser do not need inking.[1]
Selection and trimming
- If the cone is more than 1 cm long, take transverse slices from the top of the cone and towards the ectocervix, and stop when approximately 1 cm of the ectocervical portion of the cone remains.
- Cut the portion into radial or sagittal slices. Sagittal slices are made perpendicularly to the portion surface, and should be divided into at least the four quadrants.[note 1][1]
In cases where the cone is small and fragmented, try to orient the preparations and divide them if possible to obtain sagittal slices.[1]
See also: General notes on gross processing
Microscopic evaluation
Anatomic/Histologic location
Describe mucosa as squamous (or ectocervical), endocervical (generally mucinous and glandular) and/or transformation zone mucosa.
Squamous (or ectocervical) mucosa at left and endocervical (mucinous) mucosa at right, in an example with abrupt transition.
Transformation zone mucosa, consisting of a mix of stratified squamous epithelium and mucinous glands, in an example with gradual transition.
Endocervical mucosa, with mucinous columnar epithelium and mucinous glands. H&E stain
The anatomic level of the transformation zone varies:[2]
Type 1: Completely ectocervical (common under hormonal influence).
Type 2: Endocervical component but fully visible (common before puberty).
Type 3: Endocervical component, not fully visible (common after menopause).
Cervical dysplasia
edit
Look for cervical dysplasia. It is mainly seen as nuclei with hyperchromasia, coarse chromatin and irregular contours.[3]
Spectrum from normal to high grade SIL.[4]
Further information: Cervical dysplasia
Radicality
 Locations of non-radicality should be reported in relation to tissue markings (such as needles), or in terms of quadrants or corresponding to a clock face, based on the patient being in supine position. Look whether there is normal epithelium on each side of all slices where neoplasia is seen, and when the epithelium is missing in any direction, consider ordering additional serial sections or step sections.
HPV changes
Also look koilocytic changes of human papillomavirus (HPV), with such cells typically displaying:
- Nuclear enlargement (two to three times normal size).
- Irregularity of the nuclear membrane contour, creating a wrinkled or raisinoid appearance.
- A darker than normal staining pattern in the nucleus, known as hyperchromasia.
- Perinuclear cytoplasmic vacuolization ("nuclear halo").
Other findings
edit
Other common findings:
Acute cervicitis, having a largely neutrophilic intra-epithelial infiltrate.
Endocervical polyp: With endocervical epithelium and glands (mucinous columnar linings), edematous stroma and clear congestion. H&E stain.[5]
Microscopy report
If a neoplasia is found, the report should include:[1]
- The histolopathological type and degree of differentiation
- Location and extent
- Radicality
|
High-grade squamous intraepithelial lesion (CIN-2), present at 3:00 to 12:00.
All margins of excision are negative for CIN-2.
|
Example report in a normal case:
Cervix at transformation zone without significant histopathologic changes.
Negative for neoplasia/carcinoma.
|
Endometrial polyp
Author:
Mikael Häggström [note 2]
Comprehensiveness
On this resource, the following formatting is used for comprehensiveness:
- Minimal depth
- (Moderate depth)
- ((Comprehensive))
Fixation
Generally 10% neutral buffered formalin.
See also: General notes on fixation
Microscopic evaluation
The main objectives are:
- Making a diagnosis of endometrioid polyp. An endometrial polyp may be diagnosed in the presence of 2 of the following 3:
- Thick-walled vessels
- Collagenous stroma
- Epithelium on at least 3 sides
- Look for signs of atypia or malignancy.
Endometrial polyp (without atypia), with a thick-walled blood vessel in middle - typical of endometrial polyps. Glands are regular.
Endometrial polyp (without atypia), with tubal metaplasia (black arrow, showing ciliated epithelium) and a thick-walled blood vessel (white arrow). The stroma is hemorrhagic in this case.
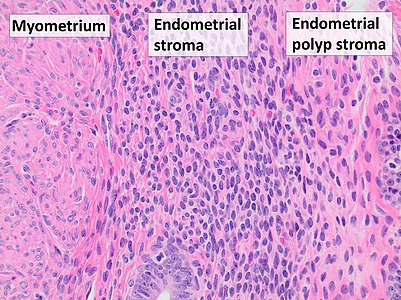
Myometrium (smooth muscle cells) versus endometrial stroma (more cellular) versus endometrial polyp stroma (more collagenous).[image 1]
Atypia (mainly seen as signs of endometrial intraepithelial neoplasia (EIN), which has the following criteria:[6]
- Architectural gland crowding
- Altered cytology relative to background glands
- Minimum size of 1 mm
- Exclusion of adenocarcinoma
- Exclusion of mimics
Mitoses should also preferably be seen.
Endometrial adenocarcinoma[7] arising in an endometrial polyp. These are most commonly endometrioid, in which case low-grade carcinoma is distinguished from hyperplasia with atypia by the presence of glandular crowding with endometrial stromal exclusion, and significant cribriform, confluent glandular, labyrinthine, papillary/villoglandular, or non-squamous solid architecture.[8]
Subserosal pedunculated uterine leiomyomas may present as endometrial polyps. They typically show smooth muscle in a fascicular pattern[9] Further information: Smooth muscle tumor
Reporting
Most importantly:
- Benign versus malignant (or presence or absence of atypia.)
- ((The size of the polyp.))
- ((The type of epithelium at both the surface and gland coverings.))
Example of a minimal report:
| Benign endometrial polyp.
|
See also: General notes on reporting
Notes
- ↑ Each slice may be individually numbered.
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
Main page
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 Monica Dahlgren, Janne Malina, Anna Måsbäck, Otto Ljungberg. Stora utskärningen. KVAST (Swedish Society of Pathology). Retrieved on 2019-09-26.
- ↑ International Federation for Cervical Pathology and Colposcopy (IFCPC) classification. References:
-. Transformation zone (TZ) and cervical excision types. Royal College of Pathologists of Australasia.
- Jordan, J.; Arbyn, M.; Martin-Hirsch, P.; Schenck, U.; Baldauf, J-J.; Da Silva, D.; Anttila, A.; Nieminen, P.; et al. (2008). "European guidelines for quality assurance in cervical cancer screening: recommendations for clinical management of abnormal cervical cytology, part 1
". Cytopathology 19 (6): 342–354. doi:10.1111/j.1365-2303.2008.00623.x. ISSN 0956-5507. PMID 19040546.
- ↑ Khaled J. Alkhateeb, M.B.B.S., Ziyan T. Salih, M.D.. HSIL / CIN II / CIN III. PathologyOutlines. Topic Completed: 29 March 2021. Minor changes: 9 February 2022
- ↑ Source image by Ed Uthman from Houston, TX, USA. Creative Commons Attribution 2.0 Generic (CC BY 2.0) license
- ↑ Anissa Ben Amor.. Cervical Ectropion. StatPearls, National Center for Biotechnology Information. Last Update: November 14, 2021.
- This book is distributed under the terms of the Creative Commons Attribution 4.0 International License
- ↑ Owings, Richard A.; Quick, Charles M. (2014). "Endometrial Intraepithelial Neoplasia
". Archives of Pathology & Laboratory Medicine 138 (4): 484–491. doi:10.5858/arpa.2012-0709-RA. ISSN 1543-2165.
- ↑ Stewart, Colin J.R.; Crum, Christopher P.; McCluggage, W. Glenn; Park, Kay J.; Rutgers, Joanne K.; Oliva, Esther; Malpica, Anais; Parkash, Vinita; et al. (2019). "Guidelines to Aid in the Distinction of Endometrial and Endocervical Carcinomas, and the Distinction of Independent Primary Carcinomas of the Endometrium and Adnexa From Metastatic Spread Between These and Other Sites
". International Journal of Gynecological Pathology 38: S75–S92. doi:10.1097/PGP.0000000000000553. ISSN 0277-1691.
- "Figures - available via license: Creative Commons Attribution 4.0 International"
- ↑ Rabban, Joseph T.; Gilks, C. Blake; Malpica, Anais; Matias-Guiu, Xavier; Mittal, Khush; Mutter, George L.; Oliva, Esther; Parkash, Vinita; et al. (2019). "Issues in the Differential Diagnosis of Uterine Low-grade Endometrioid Carcinoma, Including Mixed Endometrial Carcinomas
". International Journal of Gynecological Pathology 38: S25–S39. doi:10.1097/PGP.0000000000000512. ISSN 0277-1691.
- ↑ Mohamed Mokhtar Desouki. Uterus - Stromal tumors - Leiomyoma. pathology Outlines. Topic Completed: 1 August 2011. Revised: 15 December 2019
Image sources
Endometrial curettings
Gross processing
- Generally submit all tissue for microscopy, even larger volumes.
- Look for products of conception if indicated from referral and/or history
Microscopic evaluation
Describe the anatomic/histologic type of epithelium.
Look mainly for:
Anatomic/histologic epithelium type
(Determine the type or phase of the endometrium:) edit
 In contrast, endocervical mucosa typically consists of mucinous columnar epithelium and mucinous glands. Evaluate this like a cervical biopsy or cervical cone.
The most superficial layer of the endometrium usually consists of a simple columnar epithelium.
Postmenopausal (atrophic) endometrium: Thin epithelium, and scattered glands lined by columnar cells with small inactive nuclei, supported by a dense fibrous stroma of spindle cells. (H&E stain)
The phases of endometrium through the menstrual cycle:
Proliferative endometrium: long curving glands (G) and some stromal edema (H&E stain)
Secretory endometrium: Prominent glands (G), which have a dilated lumen and an irregular outer border stretching down into the basal compartment. In the luminal (functional) layer immune cells are readily detected (most of these are likely to be macrophages and uterine natural killer (uNK) cells), as are areas of decidualised fibroblasts (DEC) close to arterioles. (H&E stain)
If you want to specify the phase by day, then it's more accurate to state it as days past ovulation where applicable, since the follicular phase may vary substantially.
Hyperplasia, atypa and/or malignancy
- Look for signs of atypia or malignancy:
edit
Endometrial intraepithelial neoplasia (EIN), has the following criteria:[2]
- Architectural gland crowding
- Altered cytology relative to background glands
- Minimum size of 1 mm
- Exclusion of adenocarcinoma
- Exclusion of mimics
Mitoses should also preferably be seen.
Endometrial adenocarcinoma[3], most commonly endometrioid, in which case low-grade carcinoma is distinguished from hyperplasia with atypia by the presence of glandular crowding with endometrial stromal exclusion, and significant cribriform, confluent glandular, labyrinthine, papillary/villoglandular, or non-squamous solid architecture.[4]
Microscopy report
Example in a normal case:
(Endometrial curettings:
Benign proliferative endometrium and endocervical mucosa without significant histopathologic changes.) Negative for neoplasia ((or viral cytopathic changes)).
|
Endometrial thickening
Hysterectomy sampling
A regular hysterectomy grossing is performed, but with the following sampling:[5]
- 2 longitudinal sections through ecto/endocervix (1 anterior and 1 posterior)
- 2 longitudinal sections through upper endocervix/lower uterine segment (1 anterior and 1 posterior), immediately adjacent to the sections taken from the cervix
- 4 full-thickness representative sections of endomyometrium (2 anterior and 2 posterior)
- Transversely section the remaining anterior and posterior endomyometrium (~1 cm thick). Submit the entire endometrium from the lower uterine segment to the fundus, maintaining orientation.
- Submit entire fimbriae (longitudinally sectioned) and 2 representative cross-sections on each side.
Microscopic evaluation
- Look for signs of atypia or malignancy:
edit
Endometrial intraepithelial neoplasia (EIN), has the following criteria:[2]
- Architectural gland crowding
- Altered cytology relative to background glands
- Minimum size of 1 mm
- Exclusion of adenocarcinoma
- Exclusion of mimics
Mitoses should also preferably be seen.
Endometrial adenocarcinoma[7], most commonly endometrioid, in which case low-grade carcinoma is distinguished from hyperplasia with atypia by the presence of glandular crowding with endometrial stromal exclusion, and significant cribriform, confluent glandular, labyrinthine, papillary/villoglandular, or non-squamous solid architecture.[4]
Reporting
Most importantly:
- Presence or absence of atypia.
Hysterectomy
For a freshly received uterus, generally gross it fresh to include either opening it up (small specimen, no suspected malignancy seen) and/or serial sectioning, in order to let the formalin penetrate it properly. Ensure the endometrium is immersed in the formalin (such as having the serosa oriented upwards).
See also: General notes on fixation
Comprehensiveness
On this resource, the following formatting is used for comprehensiveness:
- Minimal depth
- (Moderate depth)
- ((Comprehensive))
- Other legend
<< Decision needed between alternatives separated by / signs >>
{{Common findings / In case of findings}}
[[Comments]]
Link to another page
Gross processing
Gross examination
For orientation:
- The round ligament lies anterior to the tubes and ovaries.[8]
- The peritoneum extends further down along the cervix posteriorly than anteriorly.[9] Its ends bluntly posteriorly and sharply anteriorly.[9]
When received the same day as the surgery, perform the following steps at least to serial sectioning before putting (back) in formalin, preferably with paper towels between slices, so that it fixes properly:[8]
- (Remove the adnexa.[8] Weigh the uterus without the adnexa.)
- Perform a general inspection
- Measure the 3 dimensions, including the cervix. (Also measure the length of the cervix, the maximum diameter of the cervix, and the width of the cervical os.)
- (Ink the surgical margin of the cervix for orientation, such as black on the posterior side.)
- Open the uterus by transmural radial cuts on both sides of the uterine cavity.[note 1] The cavity is sometimes be squeezed or rolled around a leiomyoma, and you'll you have to improvise and perhaps go around the leiomyoma to open the cavity properly.
- Inspect the mucosa. If any polyps: Further information: Endometrial polyp
If more extensive tumor, gross as per endometrial cancer
- Serially section through almost the entire depth of the myometrium at approximately 1 cm intervals, still keeping the specimen together.
- Measure the thickness of the mucosa and myometrium
- Inspect the myometrium. If any tumor: Further information: Smooth muscle tumor
Gross pathology of extensive adenomyosis, contrasted to normal myometrium at bottom in right image.
Gross report
Applicable in bleeding disorders, pain, leiomyoma and endometrial hyperplasia:[8]
Components:[8]
- (Shape of uterus and adnex)
- Measurements
- Mucosa, such as smooth or irregular.
- (Even the absence of) any polyps. Further information: Endometrial polyp
- Mucosal and endometrial thickness. Further information: Endometrial thickening
- (Even the absence of) any smooth muscle tumor. Further information: Smooth muscle tumor
- Example
| (A. Labeled - __. The specimen is received in formalin and consists of a resected) uterus with cervix [and bilateral fallopian tubes and ovaries]. The uterus and cervix measure __ ((cm superior to inferior)) x __ ((cm cornu to cornu)) x __ cm ((anterior to posterior,)) and weighs ___ grams. The serosa is [tan-pink and smooth]. The cervix measures ___ cm in diameter and ___ cm in length. The ectocervical mucosa is [tan-pink and smooth] and the cervical os[ is patent] and measures ___ cm in diameter. The specimen is bisected in the coronal plane. The endocervical canal is [patent and displays a tan-pink smooth mucosa]. The endometrial cavity is [triangular] and is lined by[ smooth] endometrium measuring [0.1] cm in average thickness. The myometrium measures up to ___ cm in thickness.[
- It displays __ intramural leiomyomata measuring up to __ cm in greatest diameter.
] The right ovary measures ___ cm and has a [tan-pink and smooth] capsule. Cut sections show [no gross lesions]. The right fallopian tube measures ___ cm in length and ___ cm in average diameter. The serosa [is tan-pink and smooth]. Cut sections reveal a small patent lumen and no gross lesions. The left ovary measures ___ cm and has a [tan-pink and smooth] capsule. Cut sections show [no gross lesions].The left fallopian tube measures ___ cm in length and ___ cm in average diameter. The serosa [is tan-pink and smooth]. Cut sections reveal a small patent lumen and no gross lesions. Representative sections are submitted for microscopic examination in ___ cassettes.
|
Slices for microscopy
Applicable in bleeding disorders, pain, leiomyoma and endometrial hyperplasia:[8]
Submit:[8][10]
- One, (two - at 6 and 12 o'clock), ((or four)) cross-sections from any accompanying ectocervix/endocervix (aiming to include the transformation zone). In subtotal extirpation, a cross-section is taken from the lower resection border.
- ((A transverse slice through the endocervix, possibly divided into two.))
- Endometrium and myometrium, by one slice from the front and one from the back wall of the corpus.
- {{Any mucosal parts with macroscopically abnormal appearance, including polyps.}}
- {{For any area suspicious for malignancy, submit a full cross-section of the uterine wall that includes the serosa. Use multiple contiguous cassettes if needed.}}
- {{Samples from all smooth muscle tumors >5 cm in diameter.}} Further information: Smooth muscle tumor
A specific sampling scheme is used in: Endometrial thickening
See also:
Microscopic evaluation
Look for signs of malignancy:
Cervix
edit
Look for cervical dysplasia. It is mainly seen as nuclei with hyperchromasia, coarse chromatin and irregular contours.[11]
Spectrum from normal to high grade SIL.[12]
Further information: Cervical dysplasia
edit
Other common findings:
Acute cervicitis, having a largely neutrophilic intra-epithelial infiltrate.
Endocervical polyp: With endocervical epithelium and glands (mucinous columnar linings), edematous stroma and clear congestion. H&E stain.[13]
Uterine body
(Determine the type or phase of the endometrium:) edit
 In contrast, endocervical mucosa typically consists of mucinous columnar epithelium and mucinous glands. Evaluate this like a cervical biopsy or cervical cone.
The most superficial layer of the endometrium usually consists of a simple columnar epithelium.
Postmenopausal (atrophic) endometrium: Thin epithelium, and scattered glands lined by columnar cells with small inactive nuclei, supported by a dense fibrous stroma of spindle cells. (H&E stain)
The phases of endometrium through the menstrual cycle:
Proliferative endometrium: long curving glands (G) and some stromal edema (H&E stain)
Secretory endometrium: Prominent glands (G), which have a dilated lumen and an irregular outer border stretching down into the basal compartment. In the luminal (functional) layer immune cells are readily detected (most of these are likely to be macrophages and uterine natural killer (uNK) cells), as are areas of decidualised fibroblasts (DEC) close to arterioles. (H&E stain)
If you want to specify the phase by day, then it's more accurate to state it as days past ovulation where applicable, since the follicular phase may vary substantially.
- Main pathologic findings
edit
Endometrial intraepithelial neoplasia (EIN), has the following criteria:[2]
- Architectural gland crowding
- Altered cytology relative to background glands
- Minimum size of 1 mm
- Exclusion of adenocarcinoma
- Exclusion of mimics
Mitoses should also preferably be seen.
Endometrial adenocarcinoma[15], most commonly endometrioid, in which case low-grade carcinoma is distinguished from hyperplasia with atypia by the presence of glandular crowding with endometrial stromal exclusion, and significant cribriform, confluent glandular, labyrinthine, papillary/villoglandular, or non-squamous solid architecture.[4]
Adenomyosis (endometrial glands in the myometrium). Look at these at high magnification to exclude dysplasia, as for endometrium.
Microscopy report
Examples of reports:
- Normal cases
- [note 2]
Uterus, cervix, bilateral tubes and ovaries, abdominal hysterectomy and bilateral salpingo-oophorectomy:
- Benign cervix.
- Benign inactive endometrium.
- {{Leiomyomata and adenomyosis.}}
- Benign ovaries.
- Benign fallopian tubes.
- Negative for malignancy.
|
| Microscopy of hysterectomy shows ecto and endocervix without atypia. The glands have columnar epithelium without atypia.
In the uterine cavity, there is endometrial mucosa with ordinary thickness and regularly arranged endometrial glands. (Optionally: Description of likely menstrual phase.) Sharp delimitation between endometrium and myometrium. The myometrium contains no focal changes. No evidence of malignancy.
|
- Endometrial intraepithelial neoplasia
Uterus, cervix, bilateral tubes and ovaries, hysterectomy and bilateral salpingo-oophorectomy:
- Endometrial intraepithelial neoplasia; entire endometrial/myometrial junction submitted for microscopic exam.
- Benign lower uterine segment.
- Benign bilateral tubes and ovaries.
|
Smooth muscle tumor
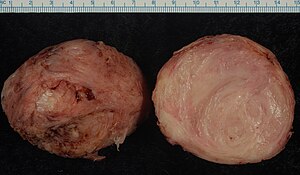 Smooth muscle tumor (in this case leiomyoma). Gross processing
Gross examination
When finding fibroids (spherical tumors with whorled pattern, which in the uterus can be presumed to be smooth muscle tumors), examine and describe:[8]
- Location. If in the uterus:
- Intramural/submucosal/subserosal (see image)
- (Posterior/anterior/right or left lateral.)
- Number of tumors, if multiple. May be described simply as "multiple".
- Size (may be described as "up to ___ cm in greatest dimension".
- (Presence or absence of any hemorrhage or necrosis.)
- ((Demarcation))
Selection
In case of hysterectomy, submit pieces from all fibroids >5 cm in diameter.[8]
Submit any macroscopically abnormal parts of the fibroids (hemorrhagic, necrotic, brittle or softening areas, and areas with blurry delimitation).[8]
For fibroids that are significantly calcified, a gross-only description as a calcified fibroid is generally sufficient, without the need to decalcify it to dissect it or sample from it.[note 3]
Microscopic examination
Distinguish leiomyoma (benign) from leiomyosarcoma (malignant) by looking at the latter's criteria:[16]
- Marked cellular atypia
- Mitoses: > 10 mitoses/10 high power fields
- Necrosis
Diagnosis of conventional leiomyosarcoma requires 2 of these 3 histologic features.[16]
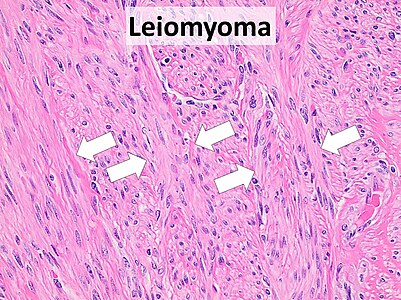
Leiomyomas typically show smooth muscle in a fascicular pattern (arrows point at fascicles).[17][image 1]
Leiomyoma, with areas where the cellularity is relatively lower (left) and higher (right).
Leiomyoma with nuclear pleomorphism, yet within benign limits.
Leiomyoma with palisading pattern
Leiomyosarcoma: Variable atypia, often with cytoplasmic vacuoles at both ends of nuclei, and frequent mitoses.[18]
Epithelioid leiomyosarcoma
Further information: Evaluation of tumors
Microscopic report
Report:
- Microscopic findings, including any visible linings
- Diagnosis or most probable one
- Any linings or borders.
Example, for a cervical polyp:
|
Polyp lined by a single layer of columnar epithelium consistent with endometrium. The interior consists of smooth muscles in a whorled pattern. No atypia. The finding is consistent with a pedunculated submucosal leiomyoma.
|
Intrauterine device
Gross processing
These are generally just visually described, with no samples for microscopic examination. Example report:
| T-shaped item with metal-coated arms and stem (Optionally: consistent with an intrauterine device with copper), with attached threads measuring 6.5 cm each.
|
Products of conception
Gross processing
Look up the gestational age of the pregnancy.
- Look for fetal tissue (fetus, fetal membranes or chorionic villi). They are generally easier to distinguish from decidua (which is maternal tissue) when fragments are put in clear fluid and shaken, with chorionic villi having a consistency like orange pulp, whereas decidua is more rubbery. The fluid may be formalin if no sample for genetic workup is needed. If chorionic villi are found, no lengthy search is needed for other kinds of fetal tissue, just a quick look for obvious ones. Membranous material is less reliable, and may still indicate further sampling. Inspect any found fetal tissue for gross anomalies. If found, submit one piece of the fetus and one piece of the placenta.[19]
Fresh chorionic villi (arrows), surrounded by decidua, at 10 weeks of gestational age.
Chorionic villi (arrow) become more characteristic when held in fluid, having a fibrillary shape
Fresh chorionic villi (left) compared to decidua (right).
Chorionic villi become more pale when fixed in formalin.
Macropathology of fixed umbilical cord, amnion and chorionic villi at 7-13 weeks of gestational age
Fresh chorionic villi in a term placenta for comparison, being more granular.
- If fetal parts are not visually found, search for diagnostic placental tissue, which is soft and shaggy or spongy (as opposed to membranous, which is likely to be decidua or blood clots).[19]
- If no fetal or placental tissue is found, all presented tissue generally needs to be submitted.
(If transported or processed together with other cases, put any chorionic villi in thin-mesh cassettes or tissue bags to limit contamination).[note 4]
Gross report
| (Labeled - products of conception.) The specimen (is received <<fresh / in formalin>>) and consists of multiple fragments of soft tan-pink decidua, blood clots and amniotic sac measuring about 5 cc in aggregate. The intact amniotic sac measures 2.3 cm in greatest dimension. Fetal tissue is identified within the amniotic sac, measuring 1.0 cm in crown-rump length. Representative sections are submitted for microscopic examination (in 1 cassette).
|
Microscopy
The most important is to detect the presence of chorionic villi.
Products of conception, with chorionic villi (arrow) among decidualized endometrium
Immature chorionic villi, having loose stroma and few capillaries.
Necrotic chorionic villi in a tubal pregnancy.
Scantly cellular decidua may look like chorionic villi, but lack the thick trophoblastic lining.
In the absence of chorionic villi, look for implantation site intermediate trophoblasts (ISITs):
ISITs, having relatively large and dark nuclei.
Histology of decidua for comparison, having smaller and brighter nuclei.
- In the absence of both chorionic villi and ISITs, preferably perform cytokeratin immunostaining of each tissue sample, such as CAM5.2.[20]
- In the absence of chorionic villi and ISITs even after staining, call the clinician, since there may be an ectopic pregnancy.
Microscopy report
Examples:
|
((Products of conception:)) Products of conception, including immature chorionic villi, corresponding to first trimester pregnancy. ((Spontaneous abortion, clinically.))
|
|
((Products of conception:)) Fragments of focally necrotic decidua and implantation site, consistent with intrauterine products of conception. Negative for chorionic villi.
|
Fallopian tube
Gross processing
(Look in the history for any intra-fallopian coils (Essure devices).)[note 5]
- For sterilization
- Measure length and average diameter of each tube
- Serially section at 3-4 mm intervals,[21] or 2-3 mm if suspected malignant (including BRCA mutation).[22] Submit
- Submit 1 (or 3) circumferential transverse sections. If the specimen is only a segment of the tube of less than <5mm((, ink the surgical cut surfaces and)) submit all tissue.[21]
Example gross report:
| (A. Labeled - __. The specimen is received in formalin and consists of) two fimbriated segments of fallopian tube measuring __ cm in length and __ cm in average diameter. On sectioning, each displays a patent lumen. No gross abnormalities are identified. The tubes are unoriented. The specimen is serially cross-sectioned and representative sections are submitted for microscopic examination in two cassettes.
|
Microscopic examination
 Fallopian tubes may be substantially edematous and congested, which can generally be attributed to surgery, in which case it does not need mention in the report. - Ensure there is at least one full cross-section from each tube, and take further samples otherwise.
- Check for patency of the lumen.
Tumor
The most common tumor of the fallopian tubes is adenomatoid tumor:[23]
An adenomatoid tumor of the fallopian tube, low magnification, displaying infiltrative-like borders.
High magnification of the same case, showing the typical[23] features of tubular spaces of varying size composed of flattened cells resembling endothelium.
Reporting
Example of a normal report in sterilization:
(Right and left fallopian tubes, ((laparoscopic)) bilateral salpingectomy:)
Complete cross-sections of histologically unremarkable fallopian tubes.
|
When included in a uterus specimen, normal tubes and ovaries may simply be mentioned as:
| Bilateral fallopian tubes and ovaries, unremarkable.
|
 Histopathology of an ectopic pregnancy in a fallopian tube, with chorionic villi and implantation site changes Further information: Products of conception . When done for ectopic pregnancy, report any rupture, either from the gross report or from microscopy, for example:
| Benign ruptured fallopian tube with ectopic products of conception, including degenerated immature chorionic villi and implantation site with fresh hemorrhage.
|
Further information: Products of conception
Notes
- ↑
- In the US, the cut goes from side to side, through the cervix and uterine cavity, keeping the anterior and posterior halves attached by a relatively thin connection left at the fundus. It is done by cutting with scissors with the blunt end in the cervix and then uterine cavity, or by a blade guided on each side by the shanks of a pair of forceps inserted through the cervix.
- In Sweden, the uterus is usually opened at the front in the midline, optionally with an incision towards each corner.
It can be done by scissors, or by inserting a probe or forceps to guide a long blade.
- ↑ The first example is used in Connecticut, and the second example is used in Sweden.
- ↑ When a fibroid has calcifications it has been there a long time, and can be assumed to be benign.
- ↑ Chorionic villi are promiscuous contaminants of other tissues, and may cause a false positive finding for another cassette containing products of conception.
- Carll T, Fuja C, Antic T, Lastra R, Pytel P (2022). "Tissue Contamination During Transportation of Formalin-Fixed, Paraffin-Embedded Blocks.
". Am J Clin Pathol 158 (1): 96-104. doi:10.1093/ajcp/aqac014. PMID 35195717. Archived from the original. .
- ↑ For a case with intra-fallopian coils in the medical records, an inability to find them on gross processing must be noted in order to raise the possibility of coil expulsion.
Main page
References
- ↑ Rao, Shalinee; Sundaram, Sandhya; Narasimhan, Raghavan (2009). "Biological behavior of preneoplastic conditions of the endometrium: A retrospective 16-year study in south India
". Indian Journal of Medical and Paediatric Oncology 30 (4): 131. doi:10.4103/0971-5851.65335. ISSN 0971-5851.
- Figure- available via license: Creative Commons Attribution 2.0 Generic
- ↑ 2.0 2.1 2.2 Owings, Richard A.; Quick, Charles M. (2014). "Endometrial Intraepithelial Neoplasia
". Archives of Pathology & Laboratory Medicine 138 (4): 484–491. doi:10.5858/arpa.2012-0709-RA. ISSN 1543-2165.
- ↑ Stewart, Colin J.R.; Crum, Christopher P.; McCluggage, W. Glenn; Park, Kay J.; Rutgers, Joanne K.; Oliva, Esther; Malpica, Anais; Parkash, Vinita; et al. (2019). "Guidelines to Aid in the Distinction of Endometrial and Endocervical Carcinomas, and the Distinction of Independent Primary Carcinomas of the Endometrium and Adnexa From Metastatic Spread Between These and Other Sites
". International Journal of Gynecological Pathology 38: S75–S92. doi:10.1097/PGP.0000000000000553. ISSN 0277-1691.
- "Figures - available via license: Creative Commons Attribution 4.0 International"
- ↑ 4.0 4.1 4.2 Rabban, Joseph T.; Gilks, C. Blake; Malpica, Anais; Matias-Guiu, Xavier; Mittal, Khush; Mutter, George L.; Oliva, Esther; Parkash, Vinita; et al. (2019). "Issues in the Differential Diagnosis of Uterine Low-grade Endometrioid Carcinoma, Including Mixed Endometrial Carcinomas
". International Journal of Gynecological Pathology 38: S25–S39. doi:10.1097/PGP.0000000000000512. ISSN 0277-1691.
- ↑ Nicole Cipriani (2020-06-22). Gross Pathology Manual. The University of Chicago Department of Pathology.
- ↑ Rao, Shalinee; Sundaram, Sandhya; Narasimhan, Raghavan (2009). "Biological behavior of preneoplastic conditions of the endometrium: A retrospective 16-year study in south India
". Indian Journal of Medical and Paediatric Oncology 30 (4): 131. doi:10.4103/0971-5851.65335. ISSN 0971-5851.
- Figure- available via license: Creative Commons Attribution 2.0 Generic
- ↑ Stewart, Colin J.R.; Crum, Christopher P.; McCluggage, W. Glenn; Park, Kay J.; Rutgers, Joanne K.; Oliva, Esther; Malpica, Anais; Parkash, Vinita; et al. (2019). "Guidelines to Aid in the Distinction of Endometrial and Endocervical Carcinomas, and the Distinction of Independent Primary Carcinomas of the Endometrium and Adnexa From Metastatic Spread Between These and Other Sites
". International Journal of Gynecological Pathology 38: S75–S92. doi:10.1097/PGP.0000000000000553. ISSN 0277-1691.
- "Figures - available via license: Creative Commons Attribution 4.0 International"
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 8.7 8.8 8.9 Monica Dahlgren, Janne Malina, Anna Måsbäck, Otto Ljungberg. Stora utskärningen. KVAST (Swedish Society of Pathology). Retrieved on 2019-09-26.
- ↑ 9.0 9.1 . General Specimen Orientation Tips. The University of Michigan (2020-01-29).
- ↑ Nicole Cipriani (2020-06-22). Gross Pathology Manual. The University of Chicago Department of Pathology.
- ↑ Khaled J. Alkhateeb, M.B.B.S., Ziyan T. Salih, M.D.. HSIL / CIN II / CIN III. PathologyOutlines. Topic Completed: 29 March 2021. Minor changes: 9 February 2022
- ↑ Source image by Ed Uthman from Houston, TX, USA. Creative Commons Attribution 2.0 Generic (CC BY 2.0) license
- ↑ Anissa Ben Amor.. Cervical Ectropion. StatPearls, National Center for Biotechnology Information. Last Update: November 14, 2021.
- This book is distributed under the terms of the Creative Commons Attribution 4.0 International License
- ↑ Rao, Shalinee; Sundaram, Sandhya; Narasimhan, Raghavan (2009). "Biological behavior of preneoplastic conditions of the endometrium: A retrospective 16-year study in south India
". Indian Journal of Medical and Paediatric Oncology 30 (4): 131. doi:10.4103/0971-5851.65335. ISSN 0971-5851.
- Figure- available via license: Creative Commons Attribution 2.0 Generic
- ↑ Stewart, Colin J.R.; Crum, Christopher P.; McCluggage, W. Glenn; Park, Kay J.; Rutgers, Joanne K.; Oliva, Esther; Malpica, Anais; Parkash, Vinita; et al. (2019). "Guidelines to Aid in the Distinction of Endometrial and Endocervical Carcinomas, and the Distinction of Independent Primary Carcinomas of the Endometrium and Adnexa From Metastatic Spread Between These and Other Sites
". International Journal of Gynecological Pathology 38: S75–S92. doi:10.1097/PGP.0000000000000553. ISSN 0277-1691.
- "Figures - available via license: Creative Commons Attribution 4.0 International"
- ↑ 16.0 16.1 Paulette Mhawech-Fauceglia, M.D.. Uterus - Smooth muscle tumors - Leiomyosarcoma. Pathology Outlines. Topic Completed: 5 December 2019. Minor changes: 11 August 2020
- ↑ Mohamed Mokhtar Desouki. Uterus - Stromal tumors - Leiomyoma. Pathology Outlines. Topic Completed: 1 August 2011. Revised: 15 December 2019
- ↑ Vijay Shankar, M.D.. Soft tissue - Smooth muscle - Leiomyosarcoma - general. Pathology Outlines. Topic Completed: 1 November 2012. Revised: 11 September 2019
- ↑ 19.0 19.1 . Gross Pathology Manual, By The University of Chicago Department of Pathology - Products of Conception. Retrieved on 2020-08-13.
- ↑ Konoplev SN, Dimashkieh HH, Stanek J (2004). "Cytokeratin immunohistochemistry: a procedure for exclusion of pregnancy in chorionic villi-negative specimen.
". Placenta 25 (2-3): 146-52. doi:10.1016/S0143-4004(03)00188-7. PMID 14972447. Archived from the original. .
- ↑ 21.0 21.1 Kerryn Ireland-Jenkin and Marsali Newman. Ovary and fallopian tube -benign setting. Royal College of Pathologists of Australasia. Retrieved on 2020-10-16.
- ↑ Crum, Christopher P.; Mckeon, Frank D.; Xian, Wa (2012). "The Oviduct and Ovarian Cancer
". Clinical Obstetrics and Gynecology 55 (1): 24–35. doi:10.1097/GRF.0b013e31824b1725. ISSN 0009-9201.
- ↑ 23.0 23.1 Nicole Riddle, Jamie Shutter. Fallopian tubes & broad ligament - Fallopian tube tumors - Adenomatoid tumor. Pathology Outlines. Topic Completed: 1 September 2013. Minor changes: 13 December 2019
Image sources
Ovary
Gross processing
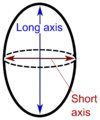 "Long" and "short" axis. [1]The ovary is cut in the longitudinal plane (through the "long axis").
Ovaries, including those with cysts, are almost never inked.
Gross report
Template:
| (A. Labeled - __. The specimen is received in formalin and consists of) an ovary measuring ___. The ovarian capsule is tan-pink and smooth. Cut sections reveal solid, white and whorled parenchyma, and no gross lesions. Representative sections are submitted for microscopic examination in __ cassettes.
|
Microscopic examination
 Signet ring cell carcinoma metastasis to the ovary, also called Krukenberg tumor: Gross pathology (top, cross-section at right) and histopathology at low and high magnification. [2] Apart from any obvious tumor, also look for signet ring cells, which is a major feature of metastatic tumors to the ovary.
Placenta
Gross processing
- Determine the shape of the placenta
- Look for any accessory lobes
- Determine the completeness of placental membranes, opacity, color and consistency (slimy/slippery?)
- Determine the point of rupture from nearest margin
- Note where the membranes are inserted
- Examine the umbilical cord
- Measure the distance between the insertion point and the nearest placental margin
- Measure the cord length and give proximal and distal diameter. In placental pathology, the proximal umbilical cord refers to the segment closest to the placenta, and distal is the segment closest to the fetus.[note 1]
- Count the number of vessels away from the insertion
- Weigh the trimmed disk, after having trimmed away the cord and membranes, and after having removed excess amounts of loose retroplacental blood clots over the maternal surface.
- Examine the fetal surface (chorionic plate):
- Note its color, in particular if it is green (often faint and tan-green, brown-green to yellow-green (which indicates meconium staining).
- Look for any pathologies including granular excrescences, subchorionic fibrin or subamniotic hemorrhage
- Look at the integrity and extent of the vasculature, including any traumatic damage. Also palpate the vasculature for any thrombosis. If a thrombus is grossly found for a live birth, the baby may have thrombosis, so the finding must immediately be reported to the clinician in care of the baby.
- Examine the maternal surface (basal plate) for completeness, adherent blood clots, depressions, calcifications and fibrin
- Take a membrane roll and cord sections, before sectioning the placenta
- With the fetal surface down on the cutting board, cut the placenta at 1cm intervals so that it can be reconstructed.
Making a fetal membrane roll.
Placental tissue after cutting, here showing severe intervillositis, with dark red and soggy tissue.
- Palpate the parencyhmal sections for areas of induration.
- Note the color of the parenchyma and describe any pale areas, cysts, thrombi, increased fibrin, calcifications and infarcts. For possible infarcts, estimate the total amount of infarcted tissue as a percentage of the placental volume. Infarction is clinically significant if it involves at least 5-10% of the placental volume.
 Gross pathology of placental disorders. [3]If you see a true knot (rather than "false knots" which are merely bulges or protuberances that may look like knots), report whether the diameter of the cord is significantly different before versus after the knot (which is a sign of constriction caused by the knot).
Tissue selection
- Distal (toward fetus) membrane roll and cross section of distal cord. It should include the area of rupture.
- Proximal (toward placenta) membrane roll and cross section of proximal cord ( 2-3 cm from insertion). They should include membranes up to the chorionic plate.
A membrane roll is created by cutting a strip, about 3 cm wide, of membrane, from the rupture site to the placental insertion. Hold the edge with forceps and roll it around the forceps, and then cut a transverse section of the roll.
Cord sections should be no thicker than 4mm.
- Placental section including fetal surface ( full thickness if possible)
- Placental section including maternal surface (full thickness if possible)
- Any lesions or abnormalities
Avoid taking placental sections near the margin. (If transported or processed together with other cases, put the placental in thin-mesh cassettes or tissue bags to limit contamination).[note 2]
Example report:
| Container A. Labeled "bladder tumor". The specimen is received in formalin and consists of multiple fragments of tan-gray, friable soft tissue measuring about __ x __ x __ cm in aggregate. The specimen is entirely submitted for microscopic examination in __ cassettes.
|
Gross report
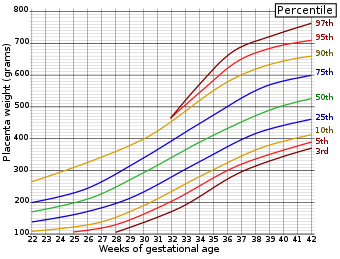 Placenta weight by gestational age. Example in a normal case:
| (A. Labeled with patient's name and medical record number. The specimen is received fresh and consists of a) placenta with attached membranes and umbilical cord. The membranes are tan-red( with a marginal insertion. The site of rupture is __ cm from the nearest placental margin. There is no accessory lobe.) The trimmed placental weight is __ gramsTemplate:Comprehensive-begin-Corresponding to the __th percentile for the gestational age)). The placental disc measures __ cm and varies in thickness from __ to __ cm. The umbilical cord is tan-pink and eccentrically inserted(, __ cm from the nearest placental margin, and measures __ cm in length, __ cm in proximal diameter and __ cm in distal diameter.) Cut sections of the cord reveal three blood vessels. The fetal surface is blue-pink, smooth with normal vasculature and << minimal / moderate / major>> subchorionic fibrin deposition. The maternal surface is complete with <<minimal / moderate / major>> physiologic calcifications. Sectioning reveals a red, spongy, homogenous parenchyma without gross lesions. (Representative sections are submitted for microscopic examination in 4 cassettes.)
KEY OF SECTIONS (example):
- 1- distal membranes and umbilical cord
- 2- proximal membranes and umbilical cord
- 3- placental section including fetal surface
- 4- placental section including maternal surface
|
Microscopic examination
- Look for inflammation, especially by the fetal surface in the intervillous spaces and around the fetal blood vessels.
Acute subchorionic intervillositis, with neutrophils (annotated) in Langhan’s layer of fibrinoid (by the fetal surface, at the base of a chorionic villus, seen at top right).
On the other hand, a small amount of intervillous neutrophils by the fetal surface like this is insignificant.
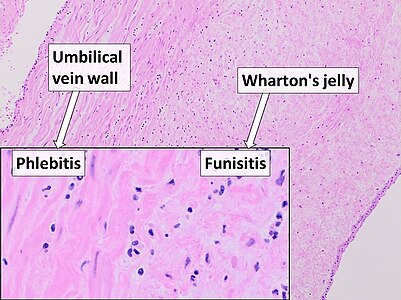
Umbilical cord: Acute phlebitis and funisitis (inflammation of a vein and the connective tissue, respectively) with neutrophils.
Acute chorioamnionitis, with neutrophils in the chorion. Also seen are fibrin thrombi, which indicate a severe fetal inflammatory response.[4]
Acute choriodeciduitis, with neutrophils seen in the chorion and decidua.
- At least if there is a suspicion of meconium in the amniotic fluid (from clinical history and/or the gross exam), look for the following histopathologic signs of it:[5]
The pigment-laden macrophages are presumably meconium-laden "meconiophages", the pathologic diagnosis can be termed "meconium histocytosis"
Other relatively common findings
Calcifications, are normal in term placentas. Report if seen in a placenta younger than 36 weeks of gestational age.[6]
Chorangiosis, an abundance of blood vessels within the chorionic villi.
Increased syncytial knotting of chorionic villi, with two knots pointed out. Causes include both hypoxia and hyperoxia.[7]
Microscopy report
Generally, also include major gross findings, such as an area of placental abruption.
Example of normal report:
(Placenta, <<vaginal/Caesarean>> delivery:)
Third trimester placenta with term villous histology. Placental weight (__ gm), at __th percentile for gestational age. Membranes without significant histopathologic changes((, negative for chorioamnionitis)). Trivascular umbilical cord, with no significant histopathologic changes((, negative for funisitis)).
|
Mild to moderate inflammation in the decidua alone can be ignored (as it is most commonly a physiological response and doesn't have a clinical significance for the fetus).
Example in a twin placenta:
Twin placenta, Caesarean section:
- Third trimester dichorionic, diamniotic twin placenta.
- Villous morphology histologically appropriate for gestational age.
- Placental weight approximately 25th percentile for gestational age.
- Two three-vessel umbilical cords.
- Negative for chorioamnionitis and funisitis.
|
Vulva
Gross processing
Generally as per skin.
Microscopic examination
 Histopathology of high-grade squamous intraepithelial lesion (HSIL) of vulva (left in image) with full thickness dysplasia, compared to normal epithelium at right. Mainly look for squamous vulvar intraepithelial lesions:
- Low-grade squamous intraepithelial lesion (LSIL):[8]
- Acanthosis, papillomatosis, and/or atypical koilocytosis in upper layers
- Usually mild atypia and mitoses, limited to the lower third of stratum spinosum and basale
- Binucleated epithelial cells
- High-grade squamous intraepithelial lesion (HSIL):[8]
- Enlarged atypical nuclei and mitoses involving middle and upper third of the epithelium.
- Also telling are atypical mitoses and/or extension in hair follicles and skin appendages.
Staging of vulvar cancer
Tumors of the vulva are staged as per the AJCC, 8th Ed:[9]
| Primary tumor (T)
|
| TNM
|
FIGO
|
Criteria
|
| TX
|
|
Primary tumor cannot be assessed
|
| T0
|
|
No evidence of a primary tumor
|
| Tisa
|
|
Carcinoma in situ (preinvasive)
|
| T1a
|
IA
|
Lesions ≤2 cm, confined to the vulva or perineum and with stromal invasion ≤1 mmb
|
| T1b
|
IB
|
Lesions >2 cm or any size with stromal invasion >1 mm, confined to the vulva or perineum
|
| T2
|
II
|
Tumor of any size with extension to adjacent perineal structures (distal third of the urethra, distal third of the vagina, anal involvement)
|
| T3
|
IVA
|
Tumor of any size with extension to any of the following proximal two thirds of the urethra, proximal two thirds of the vagina, bladder mucosa, or rectal mucosa or fixed to pelvic bone
|
| Regional lymph nodes (N)
|
| TNM
|
FIGO
|
Criteria
|
| NX
|
|
Regional lymph nodes cannot be assessed
|
| N0
|
|
No regional lymph node metastasis
|
| N1
|
|
1 or 2 regional (inguinofemoral) lymph nodes with the following features (see N1a, N1b)
|
| N1a
|
IIIA
|
1 or 2 lymph node metastases, each < 5 mm
|
| N1b
|
IIIA
|
1 regional lymph node metastasis ≥5 mm
|
| N2
|
|
Regional (inguinofemoral) lymph nodes with the following features (see N2a, N2b, N2c)
|
| N2a
|
IIIB
|
3 or more lymph node metastases, each < 5 mm
|
| N2b
|
IIIB
|
2 or more regional lymph node metastases ≥5 mm
|
| N2c
|
IIIC
|
Regional lymph node metastasis with extracapsular spread
|
| N3
|
IVA
|
Fixed or ulcerated regional lymph node metastasis
|
| Distant metastasis (M)
|
| TNM
|
FIGO
|
Criteria
|
| M0
|
|
No distant metastasis
|
| M1
|
IVB
|
Distant metastasis (including to pelvic lymph nodes)
|
Cervical cytology
Clinical information
It is not necessary to look through more than readily available reports from previous cervical cytologies.
Magnification
While being fairly new to cervical cytology, preferably start looking at a high magnification such as 20x objective (with 10x eye piece). For suspicious findings, you may magnify up to maximum. On the other hand, once the pattern feels repetitive you can try switching to a slightly lower magnification such as 10x.
Adequacy
Adequacy should always be stated, either as "Satisfactory" or "Unsatisfactory". For estimating the number of cells, determine the following:
- The area of your field of view at high power (see the Evaluation chapter)
- The total size of the relevant area on the microscope slide. A ThinPrep is about 360 mm2.
- Look at 10 representative high power fields (HPFs) within that area, and calculate the average number of cells per high power field.
 HPF example on a ThinPrep (about 360 mm 2). If 10 fields gives a total of 40 cells, it will be 4 cells per HPF. The area of this field is 0.23 mm 2. Therefore, total cellularity is estimated to be: 4 cells * 360mm 2 / 0.23mm 2 = 6260 cells.
You may count 10 fields either across the slide, or 5 fields in each direction.[10]
| Total number of cells = Average number of cells per HPF * |
Total size of area
HPF area
|
Conventional smear cellularity should be at least 8,000 cells. Liquid-based cytology cellularity should be at least 5,000 cells. Also a conventional smear is inadequate if >75% of cells are obscured by blood, exudate or air-drying artefact.[10]
Eventually you will be able to tell when most cases are adequate or inadequate without performing a detailed calculation.
Transformation zone presence
 Squamous metaplasia also counts as endocervix. Typical features are annotated. Pap stain. State whether the endocervical/transformation zone is present or absent. Count an endocervical component as present if there are 10 or more endocervical or squamous metaplastic cells.[11]
Endocervical cells can be viewed from the side as nuclear polarity (margination towards the same side as others) in a “picket-fence” configuration.
Sheets of endocervical cells have a honeycomb pattern.
In patients with previous hysterectomy, simply report glandular or squamous metaplastic cells as such, rather than stating the presence of a transformation zone, since they are likely vaginal in origin in such patients.[12]
Very common findings
For reporting, acute inflammation should be a background of ample dispersed neutrophils, and not only aggregates of neutrophils with cells or mucus.
Vaginal squamous cell with normal vaginal flora versus bacterial vaginosis on Pap stain. Normal vaginal flora (left) is predominantly rod-shaped Lactobacilli, whereas in bacterial vaginosis (right) there are clue cells, covered in bacteria. A significant amount of clue cells can be reported as "Shift in vaginal flora suggestive of bacterial vaginosis".
Candida, seen as pseudohyphae.
Main conditions to exclude or confirm
Squamous atypia, seen mainly as cells with increased nucleus/cytoplasm ratio, nuclear hyperchromasia and irregular nuclear outline.
Atypical squamous cells of undetermined significance (ASCUS), with only few slightly atypical cells
Low-grade squamous intraepithelial lesion (LSIL), here compared to an unremarkable intermediate squamous cell.
High-grade squamous intraepithelial lesion (HSIL), showing even more prominent features, and decreased cytoplasm, causing a high nuclear/cytoplasmic ratio.
Cytopathology of squamous cell carcinoma, keratinizing variant, with typical features.[13] Pap stain.
LSIL and changes consistent with human papillomavirus (HPV), which is the presence of koilocytes, which show perinuclear cavitation, binucleation, nuclear hyperchromasia, and nuclear enlargement.
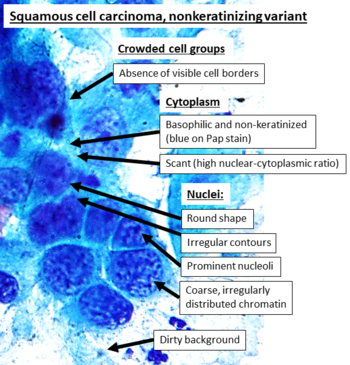 Cytopathology of squamous cell carcinoma, nonkeratinizing variant, with typical features. [14] Pap stain. Necrotic debris (dirty background) is a feature that generally makes a HSIL case "suspicious for invasive squamous cell carcinoma". [15] In contrast to the more distinct keratinizing variant, these findings are overall less specific, and most can be seen in other cancers such as adenocarcinoma as well (which, however, tends to have fine chromatin) [16]
Distinguish HPV-changes from glycogenated squamous cells. Glycogen confers a yellowish color to the cytoplasm. It can look like the perinuclear cavitation of koilocytes, but has more rounded edges.
Clinical implication
If you are uncertain of the degree of dysplasia, it can be useful to look up how much difference it will likely make for the management of the patient. You may make an Internet search for the management of abnormal cervical screening in your region (such as The ASCCP tool for management in the US). A change from close follow-up to colposcopy is not that big of a deal, but if one of the alternatives will lead to a diagnostic excision, make sure that the case is looked upon by commensurate expertise.
Report
Example in a normal case:
Cervical/endocervical ThinPrep:
- Negative for intraepithelial lesion or malignancy (NILM).
|
Male reproductive system
Phimosis
Gross processing
Generally sample one or two representative sections in a cassette, in addition to sections of any grossly visible lesions.
See also: General notes on gross processing
Microscopic evaluation
Look for:
Optionally, note any sclerosis and/or fibrosis.
Reporting
- Description of objective findings, and any suspected underlying disease.
- Presence or absence of dysplasia.
Vas deferens
Gross processing in sterilization
In sterilization:[19]
- Measure length and diameter.
- Serially section
- Submit 2 cross sections measuring 5 mm in length.
Communicate to the histology lab to section the specimen as tubular structures, in order to get proper cross-sections.
Microscopic examination in sterilization
Confirm that there is a complete cross-section from each side. Example images of normal vas deferens:
Report
Example report:
Right and left vas deferens segments, excisions:
Complete cross-sections of vasa deferentia without significant histopathologic changes.
|
Notes
- ↑ In contrast, in embryology and fetal medicine, the proximal umbilical cord refers to the segment closest to the fetus:
- Wyburn GM (1939). "The formation of the umbilical cord and the umbilical region of the anterior abdominal wall.
". J Anat 73 (Pt 2): 289-310.9. PMID 17104757. PMC: 1252509. Archived from the original. .
Harvey J. Kliman, M.D., Ph.D. (2006-10-29). The Umbilical Cord (from The Encyclopedia of Reproduction). Yale School of Medicine.
- ↑ Chorionic villi are promiscuous contaminants of other tissues, and may cause a false positive finding for a cassette containing products of conception.
- Carll T, Fuja C, Antic T, Lastra R, Pytel P (2022). "Tissue Contamination During Transportation of Formalin-Fixed, Paraffin-Embedded Blocks.
". Am J Clin Pathol 158 (1): 96-104. doi:10.1093/ajcp/aqac014. PMID 35195717. Archived from the original. .
Main page
References
- ↑ Pellerito, John; Polak, Joseph F. (2012). Introduction to Vascular Ultrasonography
(6th ed.). Elsevier Health Sciences. p. 559. ISBN 978-1-4557-3766-6.
- ↑ Nakamura, Yoshiaki; Hiramatsu, Ayako; Koyama, Takafumi; Oyama, Yu; Tanaka, Ayuko; Honma, Koichi (2014). "A Krukenberg Tumor from an Occult Intramucosal Gastric Carcinoma Identified during an Autopsy
". Case Reports in Oncological Medicine 2014: 1–5. doi:10.1155/2014/797429. ISSN 2090-6706.
- Creative Commons Attribution 3.0 Unported (CC BY 3.0) license
- ↑ Chen, Yukun; Zhang, Zhuomin; Wu, Chenyan; Davaasuren, Dolzodmaa; Goldstein, Jeffery A.; Gernand, Alison D.; Wang, James Z. (2020). "AI-PLAX: AI-based placental assessment and examination using photos
". Computerized Medical Imaging and Graphics 84: 101744. doi:10.1016/j.compmedimag.2020.101744. ISSN 08956111.
- Fig 5- available via license: Creative Commons Attribution 4.0 International.
- ↑ Kim, Chong Jai; Romero, Roberto; Chaemsaithong, Piya; Chaiyasit, Noppadol; Yoon, Bo Hyun; Kim, Yeon Mee (2015). "Acute chorioamnionitis and funisitis: definition, pathologic features, and clinical significance
". American Journal of Obstetrics and Gynecology 213 (4): S29–S52. doi:10.1016/j.ajog.2015.08.040. ISSN 00029378.
- ↑ Mandolin S. Ziadie. Placenta - Nonneoplastic placental conditions and abnormalities - Noninfectious - Meconium staining. Pathology Outlines. Topic Completed: 1 October 2011. Minor changes: 27 August 2020
- ↑ Chapter 3. Placental Calcification: Its Processes and Impact on Pregnancy, Kachewar, Sushil (2013). Calcification : processes, determinants and health impact
. New York: Nova Science Publishers, Inc. ISBN 978-1-62618-155-7. OCLC 840507829.
- ↑ Heazell AE, Moll SJ, Jones CJ, Baker PN, Crocker IP (2007). "Formation of syncytial knots is increased by hyperoxia, hypoxia and reactive oxygen species.
". Placenta 28 Suppl A: S33-40. doi:10.1016/j.placenta.2006.10.007. PMID 17140657. Archived from the original. .
- ↑ 8.0 8.1 Matthias Choschzick. Vulva, vagina & female urethra - Squamous carcinoma and precursor lesions - HPV associated SIL. PathologyOutlines. Topic Completed: 6 January 2021. Minor changes: 11 June 2021
- ↑ Amin, Mahul (2017). AJCC cancer staging manual
(8 ed.). Switzerland: Springer. ISBN 978-3-319-40617-6. OCLC 961218414.
- For access, see the Secrets chapter of Patholines.
- Copyright note: The AJCC, 8th Ed. is published by a company in Switzerland, and the tables presented therein are Public Domain because they consist of tabular information without literary or artistic innovation, and therefore do not fulfil the inclusion criterion of the Swiss Copyright Act (CopA) which applies to "literary and artistic intellectual creations with individual character" (see Federal Act on Copyright and Related Rights (Copyright Act, CopA) of 9 October 1992 (Status as of 1 January 2022)). edit
- ↑ 10.0 10.1 . Criteria for adequacy of a cervical cytology sample. EuroCytology. Retrieved on 2022-08-29.
- ↑ Cibas, Edmund S.; Ducatman, Barbara S. (2021). Cytology : diagnostic principles and clinical correlates
. Philadelphia, PA. p. 9. ISBN 978-0-323-63637-7. OCLC 1138033641.
- ↑ Ramirez NC, Sastry LK, Pisharodi LR (2000). "Benign glandular and squamous metaplastic-like cells seen in vaginal Pap smears of post hysterectomy patients: incidence and patient profile.
". Eur J Gynaecol Oncol 21 (1): 43-8. PMID 10726617. Archived from the original. .
- ↑ - Image annotated by Mikael Häggström
- Reference for entries: Gulisa Turashvili, M.D., Ph.D.. Cervix - Squamous cell carcinoma and variants. Pathology Outlines. Last author update: 24 September 2020. Last staff update: 4 April 2022.
- Source image from National Cancer Institute (Public Domain)
- ↑ - Image annotated by Mikael Häggström
- Reference for entries: Gulisa Turashvili, M.D., Ph.D.. Cervix - Squamous cell carcinoma and variants. Pathology Outlines. Last author update: 24 September 2020. Last staff update: 4 April 2022.
- Source image by Ravi Mehrotra, Anurag Gupta, Mamta Singh and Rahela Ibrahim (Creative Commons Attribution 2.0 Generic license.)
- ↑ Alrajjal A, Pansare V, Choudhury MSR, Khan MYA, Shidham VB (2021). "Squamous intraepithelial lesions (SIL: LSIL, HSIL, ASCUS, ASC-H, LSIL-H) of Uterine Cervix and Bethesda System.
". Cytojournal 18: 16. doi:10.25259/Cytojournal_24_2021. PMID 34345247. PMC: 8326095. Archived from the original. .
- ↑ Authors: Caroline I.M. Underwood, M.D., Alexis Musick, B.S., Carolyn Glass, M.D., Ph.D.. Adenocarcinoma overview. Pathology Outlines. Last staff update: 19 July 2022
- ↑ Clemmensen, Ole J.; Krogh, John; Petri, Michael (1988). "The Histologic Spectrum of Prepuces from Patients with Phimosis
". The American Journal of Dermatopathology 10 (2): 104–108. doi:10.1097/00000372-198804000-00002. ISSN 0193-1091.
- ↑ Alcides Chaux, Antonio L. Cubilla. Penis and scrotum - Inflammatory lesions - Phimosis. PathologyOutlines. Topic Completed: 1 February 2010. Revised: 13 February 2019
- ↑ . Vas Deferens (Sterilization). Gross Pathology Manual - By The University of Chicago Department of Pathology. Retrieved on 2021-08-27.
- ↑ Vas deferens image available in Public Domain. See https://patholines.org/File:Vas_deferens.jpg
Image sources
|