Upper abdominal
Contents
- 1 Liver
- 2 Liver tumor
- 3 Adrenals
- 4 Adrenal tumors
- 5 Kidney with tumor
- 6 Urinary tract stone
- 7 Pancreas
- 8 Pancreatic tumors
- 9 Pancreatic ductal adenocarcinoma
Liver
Author:
Mikael Häggström [note 1]
Comprehensiveness
On this resource, the following formatting is used for comprehensiveness:
- Minimal depth
- (Moderate depth)
- ((Comprehensive))
Tissue sampling
- Autopsy
- Liver biopsy
Fixation
Generally 10% neutral buffered formalin. Non–formalin-fixed tissue may be needed for tests such as microbiological analysis or copper quantification studies.[1]
Gross processing in autopsy
- ((Measure the distance from the liver edge to the right costal margin.))
- Inspect the color and texture of the surfaces, including external and cut surfaces. Potential pathologies:
- Look for any focal change in the liver volume, mainly any tumor. If found: Further information: Liver tumor
- Determine liver weight. The standard reference range for men is 970–1,860 g (2.14–4.10 lb)[2] and for women 600–1,770 g (1.32–3.90 lb).[3]
- Make consecutive liver slices, such as in the sagittal or coronal plane.
Gross report in autopsy
- ((Distance from the liver edge to the right costal margin.))
- Weight. If abnormally low or high, preferably include the reference range.
- Color and texture of cut surfaces
- Any focal change
Example:
| Minimal | More comprehensive | Normal ranges |
| The liver weighs ___g. | The liver is << of normal size / {{enlarged}}, at ___g. | [[Men: 970-1860 g.[2] Women 600-1770g.[3].]] |
The liver surface is <<smooth ((and glistening)) {{/deformed by small and large nodules}}((, and is <<light tan / dark brown>> in color)). << Normal/ {{/ firm}} consistency. Cut surface[note 2] is normal / << (shows normal homogeneous brown parenchyma) / {{Yellowish color, indicating steatosis}} / {{dark nutmeg similar paths, indicating congestion}}. (No focal changes.)((The liver edge is _cm below the right costal margin.))
Microscopic evaluation
A general screening includes (with further information in sections below):
- Looking at the referral/requisition form (and looking at the medical records) for particular conditions to look for or evaluate. (Perform a severity grading of previously known liver diseases.)
- Commonly, this includes to quantify any cirrhosis, at least if the patient had alcohol abuse.
- Steatosis is also common.
- Signs of acute liver failure.
- Signs of malignancy. If a tumor is found: Further information: Liver tumor
- Signs of congestive hepatopathy (indicating heart failure).
- Signs of inflammation at least around the portal triads.
Cirrhosis
Steatohepatitis with mild fibrosis (van Gieson's stain).[1]
Histopathology of steatohepatitis with moderate fibrosis (van Gieson's stain).[1]
Further information: Cirrhosis
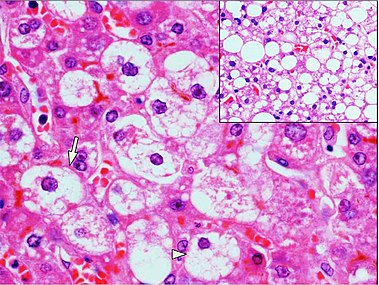
Steatosis
At least classify steatosis by severity:
Signs of acute liver failure
Lobular disarray and associated lymphocytic inflammation, acidophil acidophil body formation (arrow) and bilirubinostasis, suggesting acute hepatitis.[1]
Shock liver, showing its hallmark[4] pathologic finding centrilobular necrosis but viable periportal hepatocytes. The necrotic hepatocytes are seen as slightly more eosinophilic (red) and discohesive.
Massive hepatic necrosis: Liver cell dropout, residual hepatocytes and intact portal tract pattern.[5]
Congestive hepatopathy

- Acute hepatic congestion shows dilated sinusoidal capillaries predominantly in zone 3 of the hepatic acinus.[7]
- Typical findings of chronic hepatic congestion are atrophy of hepatocytes in zone 3, perisinusoidal edema, thrombosis and hemorrhage. Chronic congestion typically displays perivenous and perisinusoidal fibrosis, with fibrous septa that bridge central hepatic veins. In contrast, other causes of distortion and cirrhosis typically have fibrous septa predominantly between portal triads. However, nonalcoholic steatohepatitis also may show perisinusoidal fibrosis in early stages; in later stages, the fibrosis tends to be in the portal triad. Cirrhosis develops in the final stages of congestive hepatopathy. Regenerating hepatocytes tend to grow in a sleevelike pattern along portal tracts, resulting in a nodular liver with preserved portal triads and obliterated or fibrosed hepatic veins, a pattern called "reverse lobulation". This pattern can also be seen in venous obstruction due to Budd-Chiari syndrome.[8]
Hemosiderin
Hepatocyte lipofuscin alone is of no real pathologic importance and does not warrant a mention in the report.[9]
Report
- Presence of any liver disease
- (Quantification of its severity.)
- ((Even absence of hepatitis, malignancy, congestive hepatopathy and/or steatosis.))
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
- ↑ "Cut surface shows..." may alternatively be expressed as "On sectioning, the parenchyma is..."
Main page
References
- ↑ 1.0 1.1 1.2 1.3 Boyd, Alexander; Cain, Owen; Chauhan, Abhishek; Webb, Gwilym James (2020). "Medical liver biopsy: background, indications, procedure and histopathology
". Frontline Gastroenterology 11 (1): 40–47. doi:. ISSN 2041-4137.
- "This is an open access article distributed in accordance with the Creative Commons Attribution 4.0 Unported (CC BY 4.0) license"
- ↑ 2.0 2.1 Standard reference range: Molina, D. Kimberley; DiMaio, Vincent J.M. (2012). "Normal Organ Weights in Men ". The American Journal of Forensic Medicine and Pathology 33 (4): 368–372. doi:. ISSN 0195-7910.
- ↑ 3.0 3.1 Standard reference range: Molina, D. Kimberley; DiMaio, Vincent J. M. (2015). "Normal Organ Weights in Women ". The American Journal of Forensic Medicine and Pathology 36 (3): 182–187. doi:. ISSN 0195-7910.
- ↑ Ciobanu AO, Gherasim L (2018). "Ischemic Hepatitis - Intercorrelated Pathology. ". Maedica (Bucur) 13 (1): 5-11. PMID 29868133. PMC: 5972787. Archived from the original. .
- ↑ Xue, Ran; Zhu, Yueke; Liu, Hui; Meng, Qinghua (2019). "The clinical parameters for the diagnosis of hepatitis B virus related acute-on-chronic liver failure with sepsis
". Scientific Reports 9 (1). doi:. ISSN 2045-2322.
-"This article is licensed under a Creative Commons Attribution 4.0 International License" - ↑ Shah, Shailja C.; Sass, David A. (2015). "“Cardiac Hepatopathy”: A Review of Liver Dysfunction in Heart Failure
". Liver Research - Open Journal 1 (1): 1–10. doi:. ISSN 23794038.
-"This is an open access article distributed under the Creative Commons Attribution 4.0 International License (CC BY 4.0)," - ↑ . Acute Hepatic Congestion. Pathway Medicine. Retrieved on 2020-03-06.
- ↑ Wells, Michael L.; Fenstad, Eric R.; Poterucha, Joseph T.; Hough, David M.; Young, Phillip M.; Araoz, Philip A.; Ehman, Richard L.; Venkatesh, Sudhakar K. (2016). "Imaging Findings of Congestive Hepatopathy ". RadioGraphics 36 (4): 1024–1037. doi:. ISSN 0271-5333.
- ↑ . The Internet Pathology Laboratory for Medical Education. The University of Utah Eccles Health Sciences Library. Retrieved on 2020-12-18.
Image sources
Liver tumor
Author:
Mikael Häggström [note 1]
Tissue sampling
- Liver biopsy
- Autopsy: Further information: Autopsy
Gross examination
Note the following:[1]
- Whether the tumor is sell demarcated from surrounding tissue
- Whether there is visible infiltration or invasion into surrounding tissue
- Any necrosis or bleeding
Microscopic evaluation
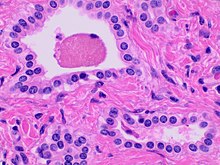
- Small to medium sized, irregularly shaped bile ducts lined by bland cuboidal epithelium (may also be flattened).
- Prominent intervening collagenous stroma.
- Bile ducts containing eosinophilic debris (may also contain inspissated bile)
Liver tumor types by relative incidence in adults in the United States.[4]
Hepatocellular carcinoma, typically resembling hepatocytes, with abundant granular eosinophilic cytoplasm.
Hepatocellular adenoma (inflammatory type pictured) may have minor atypia.[5]
Further information: Evaluation of suspected malignancies
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
Main page
References
- ↑ . General oncology. Amboss. Retrieved on 2020-01-29.
- ↑ Yang, Xiao-Yu; Zhang, Hai-Bo; Wu, Bin; Li, Ai-Jun; Fu, Xiao-Hui (2017). "Surgery is the preferred treatment for bile duct hamartomas ". Molecular and Clinical Oncology 7 (4): 649–653. doi:. ISSN 2049-9450.
- ↑ Upasana Joneja, M.D.. Liver & intrahepatic bile ducts - Developmental anomalies / cysts - Von Meyenburg complex. Pathology Outlines. Topic Completed: 23 November 2020. Minor changes: 23 November 2020
- ↑ Table 37.2 in: Sternberg, Stephen (2012). Sternberg's diagnostic surgical pathology . Place of publication not identified: LWW. ISBN 978-1-4511-5289-0. OCLC 953861627.
- ↑ Figure 7 from Bioulac-Sage, Paulette; Sempoux, Christine; Possenti, Laurent; Frulio, Nora; Laumonier, Hervé; Laurent, Christophe; Chiche, Laurence; Frédéric Blanc, Jean; et al. (2013). "Pathological Diagnosis of Hepatocellular Cellular Adenoma according to the Clinical Context
". International Journal of Hepatology 2013: 1–13. doi:. ISSN 2090-3448.
- Attribution 3.0 Unported (CC BY 3.0) license
Image sources
Adrenals
Author:
Mikael Häggström [note 1]
Comprehensiveness
On this resource, the following formatting is used for comprehensiveness:
- Minimal depth
- (Moderate depth)
- ((Comprehensive))
Main targets
Autopsy
Autopsy processing
In autopsy:
- Make a couple of cuts through the adrenal glands, such as transversal ones, and look mainly for adrenal tumors.
- ((Remove the adrenals, trim them from excessive adherent fat, and weight them. Their combined weight in an adult human ranges from 7 to 10 grams.[1]))
Adrenal cortical necrosis. Hemorrhage, fibrin thrombi and short postmortem interval indicate ante-mortem necrosis, otherwise it can be regarded as a postmortem change.[2]
Autopsy report
Normal status can be described as either:
- Adrenal glands are normal bilaterally.
- (Adrenal glands are ordinarily configured and with no definable focal changes on cut surfaces.)
- ((The adrenals are normal in size, shape and consistency, with a weight of __ grams on the right and __ grams on the left. The cortices are orange with <normal / increased / decreased thickness>. The medullae are <grey / autolyzed>.))
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
Main page
References
- ↑ O'Hare, A. Munro Neville, Michael J. (1982). The Human Adrenal Cortex Pathology and Biology – An Integrated Approach . Springer London. pp. Chapter 4: Structure of the adult cortex. ISBN 9781447113171.
- ↑ Page 120 in: Rutty, Guy (2001). Essentials of autopsy practice . London New York: Springer. ISBN 978-1-85233-541-0. OCLC 44769560.
Image sources
Adrenal tumors
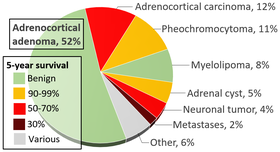
Adenoma versus carcinoma
The most common adrenal tumors are adrenocortical adenomas and carcinomas. These are most commonly distinguished by the Weiss system,[2] as follows:[3]
| Characteristic[3] | Score |
|---|---|
| High nuclear grade (enlarged, oval to lobated, with coarsely granular to hyperchromatic chromatin and easily discernible, prominent nucleoli)[4] | 1 |
| More mitoses than 5/50 high power fields | 1 |
| Atypical mitoses | 1 |
| Eosinophilic cytoplasm in >75% of tumor cells | 1 |
| Diffuse architecture of >33% of tumor | 1 |
| Necrosis | 1 |
| Venous invasion | 1 |
| Sinusoidal invasion (no smooth muscle in wall) | 1 |
| Capsular invasion | 1 |
Total score indicates:[3]
- 0-2: Adrenocortical adenoma
- 3: Undetermined
- 4-9: Adrenocortical carcinoma
Zona fasciculata versus adrenocortical adenoma. An adrenocortical adenoma typically has mild changes in comparison, including larger cells with larger and more pleomorphic nuclei with more coarse chromatin. H&E stain. [image 1]
Adrenocortical adenoma with focal high grade nuclear atypia.[5]
Adrenocortical adenoma with focal necrosis[5]
Other adrenal tumors
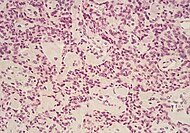
Pheochromocytoma, with typical features shown.
Reporting
For cancers, generally include a synoptic report, such as per College of American Pathologists (CAP) protocols at cap.org/protocols-and-guidelines.
Kidney with tumor
{{Renal tumor - entire article}
Urinary tract stone
Author:
Mikael Häggström [note 1]
When sent to the pathology department, these are generally sent for stone analysis to determine the chemical composition.
Gross processing
Abide by local practices for what container to use and where to leave stone specimens. Generally submit the entire specimen, or as much as you can conveniently fit in the container.
Example report:
| The specimen is received dry and consists of __ irregular fragment(s) of {{tan}} calculus measuring up to __ cm in maximum dimension. The entire specimen is submitted for spectrographic analysis. |
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
Main page
References
- ↑ Data and references for pie chart are located at file description page in Wikimedia Commons.
- ↑ Wang, Cuiping; Sun, Yang; Wu, Huanwen; Zhao, Dachun; Chen, Jie (2014). "Distinguishing adrenal cortical carcinomas and adenomas: a study of clinicopathological features and biomarkers ". Histopathology 64 (4): 567–576. doi:. ISSN 03090167.
- ↑ 3.0 3.1 3.2 Aye, Than Than; Myint, Phone; Myint, Kyar Nyo Soe (2015). "Adrenocortical Oncocytoma Presenting with Gynaecomastia ". Journal of the ASEAN Federation of Endocrine Societies 30 (1): 27–30. doi:. ISSN 08571074.
- ↑ Tito Fojo. Adrenocortical Cancer. Retrieved on 2020-07-02.
- ↑ 5.0 5.1 Gupta S, Melendez J, Khanna A (2010). "Deoxycorticosterone producing tumor as a cause of resistant hypertension.
". Case Rep Med 2010: 372719. doi:. PMID 20671982. PMC: 2909735. Archived from the original. .
- "This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited."
Image sources
- ↑ Image(s) by: Mikael Häggström, M.D. Public Domain
- Author info
- Reusing images
Pancreas
Author:
Mikael Häggström [note 1]
Comprehensiveness
On this resource, the following formatting is used for comprehensiveness:
- Minimal depth
- (Moderate depth)
- ((Comprehensive))
| Patient age | Men | Women |
| <20 years | 115g | 80g |
| 21-30 years | 115g | 125g |
| 31-40 years | 155g | 140g |
| 41-50 years | 155g | 110g |
| 51-60 years | 175g | 125g |
| >60 years | 160g | 110g |
| Overall | 160g | 120g |
In autopsy
Inspect the pancreas regarding color and consistency. ((Separate it and measure its dimensions.))
Cut it in consecutive short axis slices.[notes 2] Note the appearance of the parenchyma (and the size of the duct).
Microscopic examination
Look for pancreatic intraepithelial neoplasia and pancreatic tumors.
Stages of pancreatic intraepithelial neoplasia.[2]
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
Main page
References
- ↑ Kin, Tatsuya; Murdoch, Travis B.; Shapiro, A. M. James; Lakey, Jonathan R. T. (2017). "Estimation of Pancreas Weight from Donor Variables ". Cell Transplantation 15 (2): 181–185. doi:. ISSN 0963-6897.
- ↑ Hackeng WM, Hruban RH, Offerhaus GJ, Brosens LA (2016). "Surgical and molecular pathology of pancreatic neoplasms.
". Diagn Pathol 11 (1): 47. doi:. PMID 27267993. PMC: 4897815. Archived from the original. .
- "This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/)"
- Image title and optimization: Mikael Häggström, M.D.
Image sources
Pancreatic tumors
Microscopic evaluation
If a biopsy has been processed both for histology and cytology, make sure that their results are not contradictory.
Stages of pancreatic intraepithelial neoplasia.[1]
Relative incidences of various pancreatic neoplasm.[2]
| Cancer type | Relative incidence[3] | Microscopy findings[3] | Micrograph | ||
|---|---|---|---|---|---|
| Pancreatic ductal adenocarcinoma (PDAC) | 90% | Glands and desmoplasia | 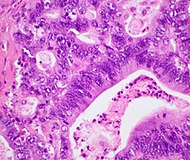
| ||
| Pancreatic acinar cell carcinoma (ACC) | 1% to 2% | Granular appearance | 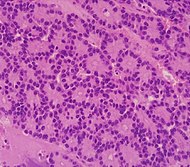
| ||
| Solid pseudopapillary tumor | Discohesive tumor nests surrounded by thin fibrous bands. |  Low and high magnification[4] | |||
| Adenosquamous carcinoma | 1% to 4%[5] | epithelial cells. | 
| ||
| Pancreatic neuroendocrine tumor | 5% | Multiple nests of tumor cells | Gastrinoma | ||
| Pre-cancer below for comparison: | |||||
| Precancer: Intraductal papillary mucinous neoplasm (IPMN) |
3% | Mucinous epithelial cells.[6] Growth within the pancreatic ducts.[7] | 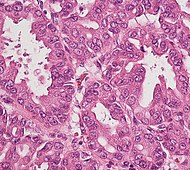
| ||
Staging
Stage pancreatic cancers as follows:[8]
| Tumor (T) | Criteria |
|---|---|
| TX | The primary tumor cannot be evaluated. |
| T0 | No evidence of cancer was found in the pancreas. |
| Tis | Carcinoma in situ. This includes:
|
| T1 | The tumor is in the pancreas only, and is ≤2 cm in greatest dimension |
| - T1a | Tumor ≤0.5 cm in greatest dimension |
| - T1b | Tumor >0.5 cm and <1 cm in greatest dimension |
| - T1c | Tumor 1–2 cm in greatest dimension |
| T2 | The tumor is in the pancreas only, and it is >2 cm and ≤4 cm in greatest dimension |
| T3 | Tumor >4 cm in greatest dimension |
| T4 | Tumor involves celiac axis, superior mesenteric artery, and/or common hepatic artery, regardless of size |
| Node (N) | Criteria |
|---|---|
| NX | The regional lymph nodes cannot be evaluated. |
| N0 | Cancer was not found in the regional lymph nodes. |
| N1 | Metastasis in 1 to 3 regional lymph nodes. |
| N2 | Metastasis in 4 or more regional lymph nodes. |
| Metastasis (M) | Criteria |
|---|---|
| M0 | No distant metastasis |
| M1 | Distant metastasis, including distant lymph nodes. Pancreatic cancer most commonly spreads to the liver, the peritoneum, and the lungs. |
Pancreatic ductal adenocarcinoma
Microscopic evaluation on biopsies
The main findings are glands formations with atypical epithelial cells and desmoplasia. If histology, clinical history and radiology are consistent, then no further immunohistochemistry or other molecular workup is needed for the diagnosis.
Make sure to also evaluate any concurrent aspiration and/or bile duct brushing.
- Further information: Pancreatic tumor
Grading
Grade as follows:[9]
- Well differentiated: > 95% tumor composed of glands
- Moderately differentiated: 50 - 95% glands
- Poorly differentiated: < 50% glands
Reporting
Example report:
A. Pancreas, core biopsy:
Comment: Note is made of concurrent aspiration cytology (DN-12-1234), which is positive for adenocarcinoma. |
- ↑ Hackeng WM, Hruban RH, Offerhaus GJ, Brosens LA (2016). "Surgical and molecular pathology of pancreatic neoplasms.
". Diagn Pathol 11 (1): 47. doi:. PMID 27267993. PMC: 4897815. Archived from the original. .
- "This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/)"
- Image title and optimization: Mikael Häggström, M.D. - ↑ Diagram by Mikael Häggström, M.D.
Source data: Wang Y, Miller FH, Chen ZE, Merrick L, Mortele KJ, Hoff FL (2011). "Diffusion-weighted MR imaging of solid and cystic lesions of the pancreas. ". Radiographics 31 (3): E47-64. doi:. PMID 21721197. Archived from the original. . - ↑ 3.0 3.1 Unless otherwise specified in boxes, reference is: "Therapeutic Implications of Molecular Subtyping for Pancreatic Cancer ". Oncology 31 (3): 159–66, 168. March 2017. PMID 28299752. Archived from the original. .
- ↑ Image by Mikael Häggström, MD.
Reference for features: Pooja Navale, M.D., Omid Savari, M.D., Joseph F. Tomashefski, Jr., M.D., Monika Vyas, M.D.. Solid pseudopapillary neoplasm. Last author update: 4 March 2022 - ↑ "Adenosquamous carcinoma of the pancreas: a case report ". Cases Journal 3 (1): 41. February 2010. doi:. PMID 20205828.
- ↑ Diana Agostini-Vulaj. Pancreas – Exocrine tumors / carcinomas – Intraductal papillary mucinous neoplasm (IPMN). Pathology Outlines. Topic Completed: 1 July 2018. Revised: 9 March 2020
- ↑ "Pathologic Evaluation and Reporting of Intraductal Papillary Mucinous Neoplasms of the Pancreas and Other Tumoral Intraepithelial Neoplasms of Pancreatobiliary Tract: Recommendations of Verona Consensus Meeting ". Annals of Surgery 263 (1): 162–77. January 2016. doi:. PMID 25775066.
- ↑ Amin, Mahul (2017). AJCC cancer staging manual
(8 ed.). Switzerland: Springer. ISBN 978-3-319-40617-6. OCLC 961218414.
- For access, see the Secrets chapter of Patholines.
- Copyright note: The AJCC, 8th Ed. is published by a company in Switzerland, and the tables presented therein are Public Domain because they consist of tabular information without literary or artistic innovation, and therefore do not fulfil the inclusion criterion of the Swiss Copyright Act (CopA) which applies to "literary and artistic intellectual creations with individual character" (see Federal Act on Copyright and Related Rights (Copyright Act, CopA) of 9 October 1992 (Status as of 1 January 2022)). - ↑ Jennifer Vazzano, D.O., M.S., Wei Chen, M.D., Ph.D.. Ductal adenocarcinoma, NOS. Pathology Outlines. Last author update: 15 June 2021