Template:Clinical pathology - entire topic
Contents
Clinical pathology
Author:
Mikael Häggström, M.D. [note 1]
Memorization-worthy:[note 2] For the most likely types of cases and/or questions that you may be responsible for, know where to find local policies and procedures, and have a good idea of whom to ask for further advice. Make sure you have access and/or contact details for whenever and wherever you are likely to need them. Preferably have at least a quick look at policies and procedures so that you have an idea of what kind of answers you will find there when needed.
Blood bank
Author:
Mikael Häggström [note 1]
If you expect to get questions regarding blood products, get a copy of the local cutoffs for approving transfusions of red blood cells, platelets and plasma, and keep it so that you can quickly look it up when needed.
Always consider if a blood product should be given as per a local precaution protocol (usually including regular check-ups of the patient).
Specific questions or requests
- Generalized dosage in adults:
- Plasma: Generally 10-15 ml/kg, and results in approximately 25-30% plasma volume replacement. Detailed calculation
- Cryoprecipitate: 1 unit for every 7-10kg of body weight. 10 units generally replaces 100-150 mg/dl. Detailed calculation
Notes
- ↑ 1.0 1.1 For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
- ↑ Further information on what is memorization-worthy or not: Learning pathology
Main page
References
Image sources
Blood compatibility testing
Author:
Mikael Häggström [note 1]
Blood typing
Upon direct testing by adding antibodies against A, B and/or Rh to patient blood, agglutination means that the patient has the antigen tested. Upon indirect testing by adding A or B antigen to patient plasma, agglutination means absence of the antigen in the patient (and thus the patient produces antibodies against it).
Identification of non-ABO antibodies
In the antibody screening procedure, an individual's plasma is added to a panel of two or three sets of red blood cells which have been chosen to express most clinically significant blood group antigens. Agglutination of the screening cells by the plasma, with or without the addition of anti-human globulin, indicates that an unexpected blood group antibody is present. If this occurs, further testing using more cells (usually 10–11) is necessary to identify the antibody. By examining the antigen profiles of the red blood cells the person's plasma reacts with, it is possible to determine the antibody's identity as follows:
The image above shows the interpretation of an antibody panel used to detect antibodies towards the most relevant blood group antigens. Each row represents "reference" or "control" red blood cells of donors which have known antigen compositions and are ABO group O. A + means that the antigen is present on the reference red blood cells, and 0 means it is absent; nt means "not tested". The "result" column to the right displays reactivity when mixing reference red blood cells with plasma from the patient in 3 different phases: room temperature, 37°C and AHG (with anti-human globulin, by the indirect antiglobulin test).[1]
- Step 1; Annotated in blue: starting to exclude antigens without reaction in all 3 phases; looking at the first reference cell row with no reaction (0 in column at right, in this case cell donor 2), and excluding (here marked by X) each present antigen where the other pair is either practically non-existent (such as for D) or 0 (presence is homozygous, in this case homozygous c).
When both pairs are + (heterozygous cases), they are both excluded (here marked by X), except for C/c, E/e, Duffy, Kidd and MNS antigens (where antibodies of the patient may still react towards blood cells with homozygous antigen expression, because homozygous expression results in a higher dosage of the antigen).[2] Thus, in this case, E/e is not excluded in this row, while K/k is, as well as Jsb (regardless of what Jsa would have shown).[note 2] - Step 2: Annotated in brown: Going to the next reference cell row with a negative reaction (in this case cell donor 4), and repeating for each antigen type that is not already excluded.
- Step 3: Annotated in purple. Repeating the same for each reference cell row with negative reaction.
- Step 4: Discounting antigens that were absent in all or almost all reactive cases (here marked with \). These are often antigens with low prevalence, and while there is a possibility of such antibodies being produced, they are generally not the type that is responsible for the reactivity at hand.
- Step 5: Comparing the remaining possible antigens for a most likely culprit (in this case Fya), and selectively ruling out significant differential antigens, such as with the shown additional donor cell type that is known to not contain Fya but contains C and Jka.
In this case, the antibody panel shows that anti-Fya antibodies are present. This indicates that donor blood typed to be negative for the Fya antigen must be used. Still, if a subsequent cross-matching shows reactivity, additional testing should be done against previously discounted antigens (in this case potentially E, K, Kpa and/or Lua).[1]
| Neutralizing substance | Antigen cancelled |
|---|---|
|
P1 |
| Saliva | H, Lea |
| Breast milk | I |
| Guinea pig urine | Sda |
When multiple antibodies are present, or when an antibody is directed against a high-frequency antigen, the normal antibody panel procedure may not provide a conclusive identification. In these cases, hemagglutination inhibition can be used, wherein a neutralizing substance cancels out a specific antigen.[2] Alternatively, the plasma may be incubated with cells of known antigen profiles in order to remove a specific antibody (a process termed adsorption); or the cells can be treated with enzymes such as ficain or papain which inhibit the reactivity of some blood group antibodies and enhance others (see table below).
Clinical implication
The following is a simplified classification for the main anti-erythrocyte antibodies, using mneumonics for the main involved antigen groups:
- Anti-A/B antibodies: These will cause immediate hemolytic transfusion reaction if the red blood cells do not have compatible antigens, even if there is no previous exposure to the antigens. Therefore, blood transfusions must always be ABO compatible.
- "Kickers"-class antibodies: Antibodies against Kidd, Kell, Rh, S and Duffy group antigens. These have a significant risk of causing hemolytic transfusion reactions when present, and therefore, patients with kickers-class antibodies should receive blood that is negative for the antigen, except for very critical situations where there is no time to find compatible blood.[3] Kickers-class antibodies generally need a previous exposure to the antigen to form, with transfusion reactions being possible upon subsequent transfusions.[2] Some patients first test positive and later test negative for a kickers-class antibody, but such patients must still be transfused with antigen-negative blood regardless.[4] They are generally of the IgG subtype, and are generally most active at 37°C. They can potentially cross the placenta and cause hemolytic disease of the newborn.
- "Limply"-class antibodies: Antibodies against Lutheran, Ii, M/N, P1, Lewis group antigens. These almost never cause clinically significant transfusion reactions (but anti-Ii antibodies are usually the type that causes cold agglutinin disease,[5] a form of autoimmune hemolytic anemia).[2] Hence, there is generally no need to find blood that is negative for the antigen for a limply-class positive patient. These antibodies are generally naturally occurring, that is, they don't require a previous exposure to the antigen to form. They are generally of the IgM class, and are generally not reactive at body temperature, but rather most active at room temperature and below.[6] They generally pose no significant risk of hemolytic disease of the newborn (as IgM class antibodies do not cross the placenta).
Following is a comparison of clinically relevant characteristics of antibodies against the main human blood group systems:[2]
| ABO | Rh | Kell | Duffy | Kidd | Lutheran | MNS | Lewis | P | Ii | |
|---|---|---|---|---|---|---|---|---|---|---|
| Most common in immediate hemolytic transfusion reactions | A | Yes | Fya | Jka | ||||||
| Most common in delayed hemolytic transfusion reactions | E,D,C | Jka | ||||||||
| Most common in hemolytic disease of the newborn | Yes | D,C | Yes | |||||||
| Commonly produce intravascular hemolysis | Yes | Yes | Yes | |||||||
| Reactive at room temperature | Yes | M,N | Lea, Leb | P1 | ||||||
| Nearly always clinically insignificant | Yes | M,N | Yes | P1 | ||||||
| Naturally occurring | Yes | Yes | M,N | Yes | Yes | Yes | ||||
| Enhanced by ficain[7] and papain[8] | Yes | Yes | Yes | Yes | P1 | Yes | ||||
| Destroyed by ficain[7] and papain[8] | Fya, Fyb | Yes | Yes | |||||||
| Displaying dosage Further information: Blood compatibility testing | Cc, Ee | Yes | Yes | Yes |
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
- ↑ Besides from C/c, E/e, Duffy, Kidd and MNS, clinically significant dosage effects is rare but not impossible for other antigens, which thus may still be considered if subsequent cross-matching is reactive.
Main page
References
- ↑ 1.0 1.1 Justin R. Rhees, M.S., MLS(ASCP)CM, SBBCM. Introduction to Antibody Identification. University of Utah, Medical Laboratory Sciences.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 Mais, Daniel (2014). Quick compendium of clinical pathology . United States: American Society for Clinical Pathology Press. ISBN 978-0-89189-615-9. OCLC 895712380.
- ↑ Pamela P. Goodell, Lynne Uhl, Monique Mohammed, Amy A. Powers (2010). "Risk of Hemolytic Transfusion Reactions Following Emergency-Release RBC Transfusion ". American Journal of Clinical Pathology 134 (2). doi:.
- ↑ Uhl, L (11 Jan 2021). Pretransfusion testing for red blood cell transfusion. UpToDate.
- ↑ "Autoimmune hemolytic anemia: current knowledge and perspectives ". Immunity & Ageing 17 (1): 38. November 2020. doi:. PMID 33292368.
- ↑ Ferdowsi S, Mohammadi S, Ahmadnezhad M, Herfat F, Rezvani A, Eshghi P (2022). "Anti-M antibody and ABO blood grouping discrepancy: a report of three cases with review of literature. ". Hematol Transfus Cell Ther 44 (2): 288-290. doi:. PMID 33358685. PMC: 9123591. Archived from the original. .
- ↑ 7.0 7.1 Hill, Ben C.; Hanna, Courtney A.; Adamski, Jill; Pham, Huy P.; Marques, Marisa B.; Williams, Lance A. (2017). "Ficin-Treated Red Cells Help Identify Clinically Significant Alloantibodies Masked as Reactions of Undetermined Specificity in Gel Microtubes ". Laboratory Medicine 48 (1): 24–28. doi:. ISSN 0007-5027. PMID 28007780.
- ↑ 8.0 8.1 Eric Ching. Questions and Answers on Proteolytic Enzymes Used in Blood Group Serology. Canadian Society for Transfusion Medicine.
Image sources
Kleihauer–Betke test
Author:
Mikael Häggström [note 1]
The Kleihauer–Betke test stains fetal red blood cells (cells containing HbF) dark reddish-pink, while adult red blood cells will be white to light pink.
Collection
EDTA-containing-tube.
Criteria for fetal cells
To count, a fetal blood cell should be:
- Stained more than approximately half of what is seen in control.
- Not be nucleated or too big (white blood cell are generally also stained).
- Not be too small.
Semi-quantification
This is done for Rh-positive mothers to estimate the severity of a suspected feto-maternal hemorrhage, which can be suspected by various clinical findings (including neonatal anemia, stillbirth, intrauterine growth restriction, hydrops fetalis, decreased or absent fetal movements, non-reassuring fetal heart rate tracing, sinusoidal fetal tracing, and fetal tachyarrythmias, placenta previa with bleeding, and placental abruption)[1].
10HPFs (in 40x) are scanned, and fetal RBCs are counted (cells per 10 HPFs, not average per HPF), and classified as:
- 0 - Negative
- 1 - Rare
- 2-5 - Few
- 6-10 - Moderate
- >10 - Abundant
Quantification
This is done for Rh-negative mothers to estimate the number of Rho(D) immune globulin vials to administer.
2000 cells are counted, in order to give a percentage calculated as:
- Fetal RBCs (given in%) = (Fetal RBC count) / (Total cell count) *100
Alternatively, an acceptable estimation of at least 2000 cells can be done by using a micrograph (or a microscopy grid) to estimate the mean number of cells in a certain area, and using the same mean to estimate the number of cells in equally sized areas:
1. Count cells (both adult and fetal) until reaching 100 cells (including each cell in square by square if using a microscopy grid). Take note of how large micrograph area (or how many grid squares) were counted (here designated as x amount), and how many fetal RBCs were counted.
2. Pick another random location (you may randomize again if it is of a significantly different cell density, but do not let your decision be influenced by the number of fetal RBCs in the area or near its edge). Count the total number of cells in the same area size (or same x number of squares), and how many of them are fetal RBCs.
3. Pick another location again, and count the number of cells in the same area size, and how many of them are fetal RBCs.
- If a count of 200-300 cells only shows 0 or 1 fetal RBC, there only needs to be 1 vial of 300 micrograms Rho(D) immune globulin, and the rest of the steps in this section can be skipped.
| Standard deviation |
Count cells in following number of areas |
|---|---|
| Up to 6 | 3 |
| 7 | 4 |
| 8 | 5 |
| 9 | 6-7 |
| 10 | 8 |
| 11 | 10 |
| 12 | 12 |
- Calculate how much the count for the second and third areas deviated from 100, and take the average thereof, which will be used as standard deviation.[note 2] If the standard deviation is higher than 12, count a total of 2000 cells regardless of areas and calculate as per formula above, and the rest of the steps can be skipped.
- Use the table at right to estimate how many areas in total you need to count in order to have a mean number of cells per area with an acceptable confidence interval.
4. After having counted the needed number of areas, calculate the average of the number of cells per area (or per x number of squares), and assume that number for the rest of the counting.
5. Divide 2000 by the number of cells per area, and round that up to know the number of areas you need to perform the next step on in order to presumably have a total of 2000 cells (including previously counted areas).
6. In those additional areas, only count the number of fetal cells per area (or x number of squares), and add that to the fetal cells from previous areas.
Fetal RBCs (given in%) = (Fetal RBC count) / (Presumable total cell count) *100
Example:
A third area of the same size yields a count of 108 cells, deviating 8 from 100. The standard deviation used[note 2] is therefore the mean of 6 and 8, which is 7. As per the table, one more area needs to be counted.
The sum of all fetal RBCs in all areas in this example is 45. The presumable total cell count is 103 * 20 = 2060. Thus:
- Fetal RBCs (given in%) = 45 / 2060 * 100 = 2.2%
Calculation of number of vials
Assuming that a vial of 300 micrograms of Rho(D) immune globulin will protect against 30 mL of fetal blood, the number of vials needed to compensate for the fetal-maternal transfusion is calculated as following, rounded up,[2] or rounded to the closest full number and then adding 1.[3]
Number of vials = Fetal RBCs in% * 1.7
For example, with 2.2% fetal RBCs, the number of vials would be 4[2] or 5[3].
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
- ↑ 2.0 2.1 Technically, the standard deviation would be calculated as the average deviation from the mean of all areas, but the simplified calculation used in this resource can be regarded to be close enough for practical purposes.
Main page
References
- ↑ Solomonia N, Playforth K, Reynolds EW (2012). "Fetal-maternal hemorrhage: a case and literature review. ". AJP Rep 2 (1): 7-14. doi:. PMID 23946896. PMC: 3653511. Archived from the original. .
- ↑ 2.0 2.1 Diann M. Krywko. Kleihauer Betke Test. StatPearls, National Center for Biotechnology Information. Last update: Last Update: January 20, 2020.
- ↑ 3.0 3.1 Practice at Danbury Hospital, Danbury, Connecticut, New England.
Image sources
Thromboelastography
Author:
Mikael Häggström, M.D. [note 1]
Interpretation
Parameters derived from thromboelastography are mainly:[1]
- R time: Time to initial clot formation (that is, amplitude deviation from baseline)
- K time: Time from initial clot formation until reaching 20 mm in amplitude
- Alpha angle (α): Angle between the baseline at initial clot formation, and a tangent line that intersects the tracing curve.
- Maximum amplitude (MA): Maximum deviation of tracing to baseline.
- A30: Amplitude 30 minutes after reaching maximum amplitude.
Following are examples of thromboelastography patterns and recommended treatments.[2][3]
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
Main page
References
- ↑ Tyler PD, Yang LM, Snider SB, Lerner AB, Aird WC, Shapiro NI (2021). "New Uses for Thromboelastography and Other Forms of Viscoelastic Monitoring in the Emergency Department: A Narrative Review. ". Ann Emerg Med 77 (3): 357–366. doi:. PMID 32988649.
- ↑ Collins S, MacIntyre C, Hewer I (2016). "Thromboelastography: Clinical Application, Interpretation, and Transfusion Management. ". AANA J 84 (2): 129–34. PMID 27311154. Archived from the original. .
- ↑ Kreitzer NP, Bonomo J, Kanter D, Zammit C (2015). "Review of Thromboelastography in Neurocritical Care. ". Neurocrit Care 23 (3): 427–33. doi:. PMID 26275677. Archived from the original. .
Image sources
Peripheral blood smear
Author:
Mikael Häggström [note 1]
Look at and comment separately on white blood cells, red blood cells and platelets. You generally don't need to aim for a perfect report, because for most purposes, a peripheral smear is a relatively simple screening test, and clinicians may opt to perform for example flow cytometry and/or a bone marrow biopsy if they want a better evaluation.
Comprehensiveness
On this resource, the following formatting is used for comprehensiveness:
- Minimal depth
- (Moderate depth)
- ((Comprehensive))
- Other legend
<< Decision needed between alternatives separated by / signs >>
{{Common findings / In case of findings}}
[[Comments]]
Link to another page
Oil immersion microscopy
This is preferred for light microscopy of peripheral blood smears in order to achieve a very high magnification. First use low or medium power to center on suspicious cells, or where red or white blood cells are best appreciated. Put a drop of immersion oil on the location and switch to the immersion objective. Then, whenever there's oil on a slide, always think twice before switching between objectives so as to avoid getting oil on any of your dry objectives (which is a bit tedious to clean off).
Red blood cells
Automated values
When available, automatic quantification of mean corpuscular volume (MCV) and red blood cell (RBC) distribution width (RDW), usually as part of CBC panel, generally decides whether you will call the sample "normocytic" versus "microcytic"/"macrocytic" and/or "anisocytotic", even if it is not clearly visible in the microscope. If automated values are not available, compare RBC sizes to lymphocyte nuclei, which should normally be the same size. If mean corpuscular hemoglobin (MCH) and mean corpuscular hemoglobin concentration (MCHC) are normal, but you still see multiple RBCs with central pallor greater than 50% of the diameter, you can report it as "Increased central pallor", and you may add "indicating iron deficiency" if it is compatible with the clinical history.
Automated values can be graded as follows:[1]
| Interpretation | Mild | Moderate | Marked |
|---|---|---|---|
| Microcytosis | MCV : 70 - 79 | MCV : 60 - 69 | MCV <60 |
| Macrocytosis | MCV : 100 - 115 | MCV : 115 - 125 | MCV >125 |
| Hypochromasia | MCH : 23 - 26 | MCH : 21 - 23 | MCH <20 |
| Anisocytosis | RDW: 14.5[2] or 16[1] - 18 | RDW : 18 - 22[1] or 26[2] | RDW > 22[1] or 26[2] |
Morphologic findings
Look for poikilocytosis (red blood cells of abnormal shapes). These are counted as a percentage of visible red blood cells:[1]
- Burr cells versus spur cells
Burr cells are distinguished from spur cells by having more equally distributed and rounded projections. They are usually artifactual. However, they may also be caused by renal insufficiency, so if this is present, a report may include "Occasional/Multiple echinocytes, consistent with renal insufficiency".
- Intraerythrocytic findings
Platelets
If CBC is performed, use count to determine whether platelets are "normal in number" or whether there is "thrombocytopenia" or "thrombocytosis". If no CBC, count platelets within a high power oil immersed field, which should normally be 8 to 20.
Large platelets are those with a diameter greater than 4 microns. Giant platelets are those with a diameter greater than 7 microns (larger than a normal red blood cell).[3] Example report:
| Numerous large and giant platelets(, suggesting an increased platelet turnover)(( such as in immune thrombocytopenic purpura. They may also be present in myeloproliferative neoplasms, myelodysplasia, and some congenital thrombocytopenia syndromes, including Bernard-Soulier syndrome and MYH9-related disorders.[3])) |
In thrombocytopenia from automatic counting, look in particular for:
- Clumping of platelets (which can cause a falsely low automatic platelet count). If present, check with the lab if it was sent in EDTA (which may cause artefactual clumping) and ask to have a blood sample sent in sodium citrate instead. Also, look for satellitosis (platelets attached around white blood cells).
- Schistocytes among red blood cells.
White blood cells
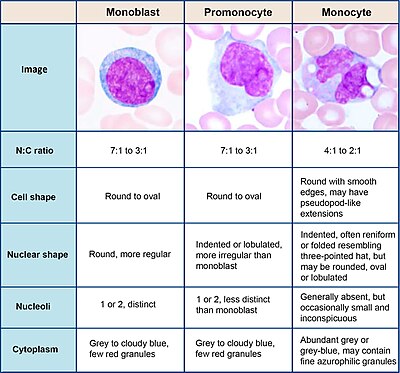
Look for:
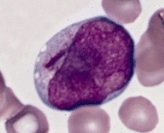
Blast cells, generally having large nucleus, immature chromatin, a prominent nucleolus, scant cytoplasm and few or no cytoplasmic granules. This example has an Auer rod (to the left of the nucleus). Further information: Suspected blasts on peripheral blood smear
Hypersegmented neutrophils. This is abnormal when more than half of neutrophils have at least 4 segments, or more than 5% of neutrophils have more than 5 segments.[4]
In patients with known chronic lymphocytic leukemia, estimate the percentage of prolymphocytes, which are medium-sized lymphocytes with prominent nucleoli.[5] A percentage of less than 5% can be reported as such.
When smudge cells constitute more than 10% of white blood cells, or in patients with CLL, a separate smear with a drop of serum albumin to every four or five drops of blood should be made to stabilize cell membranes.[6] A semi-quantification of different cell lines should then be made on the albumin slide, whereas white and red blood cell morphology should still be made on the original slide, since it is altered by albumin.
Report
Example report:
| Normochromic normocytic red blood cells. Red blood cells show <normal morphology / anisopoikilocytosis with occasional ___>. [[If thrombocytopenia, also add "Schistocytes are not significantly increased" if applicable.]]
{{Leukocytosis with neutrophilia / lymphocytosis.}} White blood cells show no left shift or blasts. Platelets show no evidence of clumping, and show normal granularity. (Causes of the above findings include ___.) |
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
Main page
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Unless otherwise specified in table, reference is:
- . Hong Kong Medical Technology Association - Quality Assurance Programme - Haematology and Serology, Prepared by HKMTAQAP Haematology & Serology Panel on November 2002.. - ↑ 2.0 2.1 2.2 . High RDW level in the blood. MrLabTest. Last update: 12/01/2021
- ↑ 3.0 3.1 Teresa Scordino (2016-12-02). Giant platelets. American Society of Hematology.
- ↑ Glassy, Eric (1998). Color atlas of hematology : an illustrated field guide based on proficiency testing . Northfield, Ill: College of American Patholgists. ISBN 978-0-930304-66-9. OCLC 40976106.
- ↑ . prolymphocytes in PLL. American Society of Hematology (2013-07-16).
- ↑ Fredrick L. Kiechle. Q & A. CAP Today. June 2010
Image sources
Platelet aggregation study
Author:
Mikael Häggström [note 1]

In a platelet aggregation study, the aggregation process is started by different agonists (ADP, epinephrine etc.) and the aggregation pattern can usually conform into any of the following patterns:
| ADP | Epinephrine | Collagen | Ristocetin | |
|---|---|---|---|---|
| P2Y receptor defect[2] (including Clopidogrel) | Decreased | Normal | Normal | Normal |
| Adrenergic receptor defect[2] | Normal | Decreased | Normal | Normal |
| Collagen receptor defect[2] | Normal | Normal | Decreased or absent | Normal |
| Normal | Normal | Normal | Decreased or absent | |
|
Decreased | Decreased | Decreased | Normal or decreased |
| Storage pool deficiency[3] | Absent second wave | Partial | ||
| Aspirin or aspirin-like disorder | Absent second wave | Absent | Normal | |
Further reading
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
Main page
References
- ↑ Jiang, L.; Xu, C.; Yu, S.; Liu, P.; Luo, D.; Zhou, Q.; Gao, C.; Hu, H. (2013). "A critical role of thrombin/PAR-1 in ADP-induced platelet secretion and the second wave of aggregation ". Journal of Thrombosis and Haemostasis 11 (5): 930–940. doi:. ISSN 15387933. PMID 23406164.
- ↑ 2.0 2.1 2.2 2.3 2.4 Borhany, Munira; Pahore, Zaen; ul Qadr, Zeeshan; Rehan, Muhammad; Naz, Arshi; Khan, Asif; Ansari, Saqib; Farzana, Tasneem; et al. (2010). "Bleeding disorders in the tribe: result of consanguineous in breeding ". Orphanet Journal of Rare Diseases 5 (1). doi:. ISSN 1750-1172.
- ↑ 3.0 3.1 . Why Perform Platelet Aggregation?. Helena Biosciences. 2015
Image sources
Bone marrow
Author:
Mikael Häggström [note 1]
Autopsy
Using pliers or similar tool, squeeze some bone marrow from a rib.
Microscopic evaluation
- Confirm trilineage hematopoiesis (see image).
- Look for apparent cellular atypia or decrease of cellular diversity.
Report
Example in a normal case:
| Bone marrow from rib (or other location if applicable): Trilineage hematopoiesis. There is no evidence of malignancy. |
Bone marrow biopsy
Gross processing
- Ensure that the biopsy is properly fixed (generally at least 2 hours).
- Measure length and diameter of each fragment.
- If the biopsy is received in a zinc-containing fixative, rinse it for about 2 minutes, such as placing the cassette in a container under running water (and block any sink enough so that the cassette won't float away).
- Put in decalcifying solution, preferably using a type that is optimal for performing subsequent immunohistochemistry. Generally decalcify the specimen for about 15 minutes initially, and palpate the specimen to check whether it is still firm. If it is, decalcify another 5-10 minutes and check again. Rather have it a little bit too soft than a little bit too firm. Be careful to follow grossing guidelines for bone marrows, since even a small aberration in the processing is likely to be noticed as creating artifacts.
- Gross report example
| The specimen is received in AZF solution and consists of __ core needle bone marrow biopsy fragment(s) measuring __ cm in length and 0.2 cm in diameter. The specimen is entirely submitted for microscopic examination in one cassette following decalcification. |
Microscopic evaluation
Evaluate the following:
- Bone marrow aspirate
Find a location where bone marrow cells can be seen individually, preferably with minimal peripheral blood among them. Sometimes it is between trabeculae and sometimes it is in the surrounding area. Perform a differential count by counting 500 cells[1] and dividing the numbers by 5 to get their percentages. Don't count cells that don't have a cytoplasm or nucleus. If you receive several slides, count about the same number of cells from each slide, but don't count cells from slides where the cell types are more indistinct.
- Myeloid/erythroid ratio, usually defined as the ratio between the number of neutrophil granulocytes and precursors versus the number of erythroblasts. Depending on source, the lower limit of the normal range for this ratio bone marrow aspirate smears in adults is 1 to 2, and the upper limit is 5 to 8.[2] In histologic sections, the normal range is 1.5 – 3.0.[2] Some hematologists also include eosinophils, basophils and monocytes, as well as their precursors, in the myeloid number, but this has only a minor effect on the M:E ratio in normal individuals.[2] Still, if you are to calculate the value for a senior, check their preference first.
In a bone marrow count, nucleated red blood cells count into the total percentage of cells (but does not count into percentage of white blood cells in peripheral blood).
- Bone marrow biopsy, H&E stain
- Adequacy: There should preferably be 5 intertrabecular spaces.
- Cellularity: This is a rather rough estimation of the area of hematopoietic cells divided by the area of all intertrabecular matter, which otherwise mainly consists of fat cells and a small interstitial space. Areas of bone, hemorrhage or artifacts are not counted. In patients 20-80 years, the percentage should be about 100 minus age, such as 40% in a 60 year old patient.
- Thrombopoiesis: There are normally about 3 megakaryocytes per 40x field.
- Look for any granuloma or cancer metastasis
- Bone marrow biopsy, other stains
Generally including:

- Iron stain: Look at approximately 100 cells and semi-quantify the presence of any ring sideroblasts.
- Reticulin to grade the amount of fibrosis.
- CD3 and CD20 to highlight T and B cells, respectively.
Microscopic report
Example template:
On this resource, the following formatting is used for comprehensiveness:
- Minimal depth
- (Moderate depth)
- ((Comprehensive))
- Other legend
<< Decision needed between alternatives separated by / signs >>
{{Common findings / In case of findings}}
[[Comments]]
Link to another page
|
SOURCE:
CLINICAL INFORMATION:
DIAGNOSIS:
BONE MARROW, {{POSTERIOR ILIAC CREST,}} ASPIRATE AND BIOPSY:
GROSS DESCRIPTION:
MICROSCOPIC DESCRIPTION: PERIPHERAL SMEAR:
MORPHOLOGY:
BONE MARROW ASPIRATE:
CELLULARITY ESTIMATE: Adequate. / Hypocellular and hemodilute. / Hypercellular. / Too few cells for morphologic evaluation. MARROW DIFFERENTIAL
ERYTHROPOIESIS: Maturing. GRANULOPOIESIS: Maturing. MEGAKARYOCYTES: Present. LYMPHOCYTES: Mature. PLASMA CELLS: Rare without atypia. BONE MARROW TOUCH PREPARATION: _ BONE MARROW BIOPSY:
SPECIMEN ADEQUACY:
CELLULARITY:_
Decalcified bone marrow biopsy demonstrates ____________________ BM Erythropoiesis _ BM Cellularity _ BM Megakaryocytes _ BM Myelopoiesis _ BONE MARROW IRON STAIN: Storage iron _/4 on aspirate smear with / without ring sideroblasts. SPECIAL STAINS:
FLOW CYTOMETRY:
COMMENT:
|
Cite error: <ref> tags exist for a group named "note", but no corresponding <references group="note"/> tag was found, or a closing </ref> is missing
- ↑ Abdulrahman AA, Patel KH, Yang T, Koch DD, Sivers SM, Smith GH (2018). "Is a 500-Cell Count Necessary for Bone Marrow Differentials?: A Proposed Analytical Method for Validating a Lower Cutoff. ". Am J Clin Pathol 150 (1): 84-91. doi:. PMID 29757362. Archived from the original. .
- ↑ 2.0 2.1 2.2 BJ. Bain (2017-02-19). Chapter 5: Pathology of the marrow. Basicmedical Key.
- ↑ Salama M, Teruya-Feldstein J, Kremyankaya M. Atlas of Diagnostic Hematology. Philadelphia, PA: Elsevier; 2021.