Intestine with tumor
Author:
Mikael Häggström [note 1]
Contents
Comprehensiveness
On this resource, the following formatting is used for comprehensiveness:
- Minimal depth
- (Moderate depth)
- ((Comprehensive))
Gross examination

- Orientation: Determine the proximal (oral) and distal (aboral) parts of the specimen if possible.[1]
- Measure the length of the entire specimen.[1] (Also measure diameter.)
- For extraperitoneal segments such as the distal and posterior rectum, rate the completeness of the attached mesocolon, with following example for mesorectal excisions:[2]
| Volume | Defects | Cone shape[notes 1] | Circumferential resection margin | |
|---|---|---|---|---|
| Complete | Smooth, intact | Less than 5 mm | No | Smooth, regular |
| Almost complete | Moderate volume | There is no visible muscularis propria | Moderate | Irregular |
| Incomplete | Small volume | Up to the muscularis propria | Yes | irregular |
- (Surfaces that appear to overlie a known or suspected tumor are preferably inked, even for serosal surfaces.) (Also take a photo of surfaces overlying a tumor.)
- Describe the serosa, and whether there are any suspected tumor breakthroughs hereof.[1]
- Initial intestinal opening can be:
- A longitudinal cut opposite to the tumor if it is relatively demarcated (by sight and/or palpation).
- Transverse (cross-sectional) slicing, until reacing the tumor, particularly for circumferential tumors.
- Measure the distances to the proximal and distal surgical margins.[1]
- Note any accompanying polyps.[1] See Colorectal polyp
- Evaluate the following either before or after slicing it up:[1]
- Tumor size
- The proportion of the circumference involved
- Any significant stricture of the lumen
- Serially section the tumor, either by transverse or longitudinal sectioning.[1]
- Review each slice and note the depth (in terms of anatomic layer, possibly with rough percentage thereof) and distance to the serosa or transverse resection margin for any tumor invasion and/or infiltration.[1]
- Carefully go through included mesentery for lymph nodes. A consensus standard is to find at least 12 lymph nodes from colon specimens.[3] Further information: Lymph nodes
Tissue selection
Should include:[1]
- The tumor slices that show the deepest penetration. The slices should include tumor relation to the serosa or resection margin, as well as adjacent normal mucosa. If transported or processed together with other cases, put the tumor slices in thin-mesh cassettes or tissue bags to limit contamination.[note 2]
- Proximal and distal resection margin, respectively. Take transverse slices except if the tumor is within 2 cm from the margin, in which case it is advisable to take slices perpendicular to that margin, including both the tumor border and the resection margin. Ink the margins when taking perpendicular sections(, but this is optional when taking transverse slices.)
- Take slices of any other suspicious findings
- Take a slice of the normal intestinal wall
- Take slices from any adherent structures and/or organ parts
- Lymph nodes.
Gross report
Legend:
<< Decision needed between alternatives separated by / signs >>
{{Common findings / In case of findings}}
Organs or important regions are in bold in the report example, but does not need to be in an actual report.
The gross report include:[1]
- Dimensions of entire sample, as well as for the tumor
- Distance to proximal and distal resection margins
- Depth of tumor invasion and/or infiltration, preferably with distance to circumferential margin. It can include a "macroscopic staging" as per the #Staging section below.
- Completeness of the attached mesocolon for extraperitoneal segments.
Minimal reporting example:
| 11.5 cm long intestinal sample. 23 mm from distal margin is a polypoid irregular brown tumor, measuring 58 x 39 x 28 mm. 18 mm to circumferential margin. |
Comprehensive example for a rectal resection:
|
See also: General notes on reporting
Microscopic evaluation of colorectal tumors
Determine tumor type and differentiation.

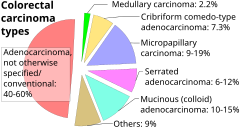
Intestinal tumors are generally colorectal carcinomas, specifically colorectal adenocarcinoma, so each evaluation can primarily focus on whether such is the case. Other cancer types are displayed in section on small intestinal tumors below
Microscopy criteria for colorectal adenocarcinoma
- A lesion at least "high grade intramucosal neoplasia" (high grade dysplasia) has:
- Severe cytologic atypia[5]
- Cribriform architecture, consisting of juxtaposed gland lumens without stroma in between, with loss of cell polarity. Rarely, they have foci of squamous differentiation (morules).[5]
- This should be distinguished from cases where piles of well-differentiated mucin-producing cells appear cribriform. In such piles, nuclei show regular polarity with apical mucin, and their nuclei are not markedly enlarged.[5]
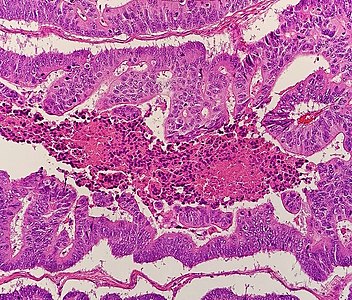
- Invasive adenocarcinoma commonly displays:
- Varying degrees of gland formation with tall columnar cells.[5]
- Frequenty desmoplasia.[5]
- Dirty necrosis, consisting of extensive central necrosis with granular eosinophilic karyorrhectic cell detritus.[5][6] It is located within the glandular lumina,[6] or often with a garland of cribriform glands in their vicinity.[5]
It may also show lymphovascular invasion.
Further reading: Colorectal adenocarcinoma
Staging
Determine depth of growth and/or infiltration. In case of cancer, stage by the AJCC or TNM system:
| AJCC stage[7] | TNM stage[7] | TNM stage criteria[7] |
|---|---|---|
| Stage 0 | Tis N0 M0 | Tis: Tumor confined to mucosa; cancer-in-situ |
| Stage I | T1 N0 M0 | T1: Tumor invades submucosa |
| T2 N0 M0 | T2: Tumor invades muscularis propria | |
| Stage II-A | T3 N0 M0 | T3: Tumor invades subserosa or beyond (without other organs involved) |
| Stage II-B | T4a N0 M0 | T4a: Tumor perforates the visceral peritoneum |
| Stage II-C | T4b N0 M0 | T4b: Tumor invades adjacent organs |
| Stage III-A |
|
|
| Stage III-B |
|
|
| Stage III-C |
|
|
| Stage IVa | any T, any N, M1a | M1a: Metastasis to 1 other part of the body beyond the colon, rectum or regional lymph nodes. Any T, any N. |
| Stage IVb | any T, any N, M1b | M1b: Metastasis to more than 1 other part of the body beyond the colon, rectum or regional lymph nodes. Any T, any N. |
| Stage IVc | any T, any N, M1c | M1c: Metastasis to the peritoneal surface. Any T, any N. |
- Further information: Evaluation of tumors
Microscopic evaluation of small intestinal tumors

Consider mainly the most common tumors:
- Malignant small intestinal tumors (60% of cases)[9], see diagram.
- Leiomyoma, approximately 15%, generally evaluated as a Spindle-cell tumors of the midgut.[10]
- Adenoma, approximately 7%.[10]
- Lipoma, approximately 5%.[10]
| Type | Evaluation | Image |
|---|---|---|
| Adenocarcinoma | As per colorectal cancer in previous section | 
|
| Neuroendocrine tumors of the midgut | 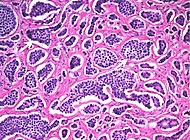
| |
| Spindle-cell tumors of the midgut | Generally perform c-Kit immunohistochemistry to detect any gastrointestinal stromal tumor.[11] | |
| Lipoma | As per lipomatous tumor | 
|
- Further information: Evaluation of tumors
Microscopy report
It should include:[12]
- Whether the resection is radical
- Any breakthrough of the serosa and/or resection margin
- Number of lymph nodes found.
- Number of them with metastases and/or periglandular growth. Look extra close at tumors with tattoo ink.
- AJCC or TNM stage if applicable
Example:
| Colon, segmental colectomy: Tubulovillous adenoma (5.0 cm in greatest dimension) with high-grade dysplasia. 18 lymph nodes negative for carcinoma. |
For cancers, generally include a synoptic report, such as per College of American Pathologists (CAP) protocols at cap.org/protocols-and-guidelines.
See also: General notes on reporting
Notes
- ↑ A cone shape is a tapered distal end, generally by removed mesorectum. It is applicable mainly to low anterior resection (LAR) specimens.
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
- ↑ Colorectal adenocarcinoma (the most common colon tumor) is a promiscuous contaminant of other tissues.
- Carll T, Fuja C, Antic T, Lastra R, Pytel P (2022). "Tissue Contamination During Transportation of Formalin-Fixed, Paraffin-Embedded Blocks. ". Am J Clin Pathol 158 (1): 96-104. doi:. PMID 35195717. Archived from the original. .
Main page
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 1.8 1.9 Unless otherwise specified, reference is: Monica Dahlgren, Janne Malina, Anna Måsbäck, Otto Ljungberg. Stora utskärningen. KVAST (Swedish Society of Pathology). Retrieved on 2019-09-26.
- ↑ Delibegovic, Samir (2017). "Introduction to Total Mesorectal Excision ". Medical Archives 71 (6): 434. doi:. ISSN 0350-199X.
- ↑ Wong SL (2009). "Lymph node counts and survival rates after resection for colon and rectal cancer. ". Gastrointest Cancer Res 3 (2 Suppl): S33-5. PMID 19461921. PMC: 2684729. Archived from the original. .
- ↑ Kang, Hakjung; O’Connell, Jessica B.; Leonardi, Michael J.; Maggard, Melinda A.; McGory, Marcia L.; Ko, Clifford Y. (2006). "Rare tumors of the colon and rectum: a national review ". International Journal of Colorectal Disease 22 (2): 183–189. doi:. ISSN 0179-1958.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 Robert V Rouse. Adenocarcinoma of the Colon and Rectum. Stanford University School of Medicine. Original posting/updates: 1/31/10, 7/15/11, 11/12/11
- ↑ 6.0 6.1 Li, Lianhuang; Jiang, Weizhong; Yang, Yinghong; Chen, Zhifen; Feng, Changyin; Li, Hongsheng; Guan, Guoxian; Chen, Jianxin (2014). "Identification of dirty necrosis in colorectal carcinoma based on multiphoton microscopy ". Journal of Biomedical Optics 19 (6): 066008. doi:. ISSN 1083-3668.
- ↑ 7.0 7.1 7.2 . Colorectal Cancer: Stages. Cancer.net (American Society of Clinical Oncology). Retrieved on 2019-09-26. Approved by the Cancer.Net Editorial Board, 11/2018. In turn citing:
Amin, Mahul B.; Greene, Frederick L.; Edge, Stephen B.; Compton, Carolyn C.; Gershenwald, Jeffrey E.; Brookland, Robert K.; Meyer, Laura; Gress, Donna M.; et al. (2017). "The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging ". CA: A Cancer Journal for Clinicians 67 (2): 93–99. doi:. ISSN 00079235. - ↑ Qubaiah, O.; Devesa, S. S.; Platz, C. E.; Huycke, M. M.; Dores, G. M. (2010). "Small Intestinal Cancer: a Population-Based Study of Incidence and Survival Patterns in the United States, 1992 to 2006 ". Cancer Epidemiology Biomarkers & Prevention 19 (8): 1908–1918. doi:. ISSN 1055-9965.
- ↑ Template:Cite
- ↑ 10.0 10.1 10.2 Beatriz Rodriguez-Vigil, MD , Manuel Lamas, MD , Arturo Alvarez-luque, MD (2006-06-03). Small bowel findings reveal tumor spectrum. diagnosticimaging.com.
- ↑ Lee, So Jung; Hwang, Chung Su; Kim, Ahrong; Kim, Kyungbin; Choi, Kyung Un (2016). "Gastrointestinal tract spindle cell tumors with interstitial cells of Cajal: Prevalence excluding gastrointestinal stromal tumors ". Oncology Letters 12 (2): 1287–1292. doi:. ISSN 1792-1074.
- ↑ 12.0 12.1 12.2 Monica Dahlgren, Janne Malina, Anna Måsbäck, Otto Ljungberg. Stora utskärningen. KVAST (Swedish Society of Pathology). Retrieved on 2019-09-26.
Image sources