Invasive ductal carcinoma
Author:
Mikael Häggström [note 1]
Contents
Comprehensiveness
On this resource, the following formatting is used for comprehensiveness:
- Minimal depth
- (Moderate depth)
- ((Comprehensive))
Gross examination
As per:
or mastectomy.
Microscopic evaluation
Characteristics
Low power:[1]
- Sheets, nests, cords or individual cells, but generally ratherplump tumor nests (rather than the single-file linear pattern of invasive lobular carcinoma)
- Prominent tubular formations in well differentiated tumors, but absent when poorly differentiated
- The stroma is usually desmoplastic
High power typically shows tumor cells that are more pleomorphic than in lobular carcinoma.[1]
Differential diagnosis
Invasive versus in situ
In invasive ductal carcinoma, malignant cells have penetrated the basement membrane, in contrast to ductal carcinoma in situ. In uncertain cases, use immunohistochemistry stain for calponin (has the highest sensitivity) and p63 (has the highest specificity).
Invasive ductal carcinoma with tubular features can look like benign tubules, but the myoepithelial marker[note 2] calponin and p63 shows no surrounding myoepithelial cells.
Immunohistochemistry for calponin in ductal carcinoma in situ, highlighting myoepithelial cells around all tumor cells.
Fibroadenoma may have small cell clusters, but lacks the cellular atypia of invasive ductal carcinoma.
In case of both invasive and in situ carcinoma, separately describe the in situ component. Report as "extensive intraductal component" (EIC) if DCIS is occupying 25% or more of the area encompassed by the invasive tumor and DCIS present in grossly normal adjacent breast tissue.[2]
Ductal versus lobular
- Invasive lobular carcinoma typically has single files of tumor cells rather than duct-forming tumor cells. In uncertain cases, stain for E-cadherin and p120:
Invasive lobular carcinoma, in this case with a targetoid pattern
p120 has cytoplasmic staining in invasive lobular carcinoma (shown), but has membranous staining in invasive ductal carcinoma
Staging
Stage by the TNM system as follows in sections below.
Also, look for any angiolymphatic invasion. If present, check whether it reaches outside the tumor, and if so, how far.[3] Give greatest dimension (,or 3 dimensions, generally by adding up the estimated thicknesses of involved slices)).[3]
Primary Tumor (T)Tumor – Depends on the tumor at the primary site of origin, as follows:[4]
Regional Lymph Nodes (N)Lymph Node: The lymph node values depend on the number, size and location of breast cancer cell deposits in various regional lymph nodes, such as the armpit (axillary lymph nodes), the collar area (supraclavicular lymph nodes), and inside the chest (internal mammary lymph nodes.)[5][6] Each stage is as follows:[4]
Critical numbers of involved nodes: 1-3, 4-9, and 10 and over. Note any extranodal extension.[3]
Distant Metastases (M)
Overall stageA combination of T, N and M, as follows:[4]
|
- Further information: Evaluation of tumors
Grading
The Nottingham system[7] is recommended for breast cancer grading.[8] The Nottingham system is also called the Bloom–Richardson–Elston system (BRE)[9], or the Elston-Ellis modification[10] of the Scarff-Bloom-Richardson grading system.[11][12] It grades breast carcinomas by adding up scores for tubule formation, nuclear pleomorphism, and mitotic count, each of which is given 1 to 3 points. The scores for each of these three criteria are then added together to give an overall final score and corresponding grade as follows.
Tubule formation
The overall appearance of the tumor is considered.[13]
- 1 point: tubular formation in more than 75% of the tumor
- 2 points: tubular formation in 10 to 75% of the tumor ("moderate")
- 3 points: tubular formation in less than 10% of the tumor ("little or none")
Nuclear pleomorphism
Such as nuclei being larger, darker, or irregular/pleomorphic. Note: The cancer areas having cells with the greatest atypia should be evaluated.
- 1 point: Nuclei are small or mildly increased in size compared to normal breast epithelial cells. They have uniform nuclear chromatin and only mild pleomorphism.
- 2 points: nuclei with moderate variation in size and shape. Cells are larger than normal (usually 1.5 - 2 times larger)[14], display open vesicular nuclei, have visible nucleoli.
- 3 points: nuclei with marked variation in size and shape. Cells display vesicular nuclei, often prominent nucleoli. Often very large and bizarre cells.
Mitotic count
Mitotic figures are counted only at the periphery of the tumor, and counting should begin in the most mitotically active areas, and in at least 10 high-power fields (HPFs). If you know that the area of your high power field is about 0.2mm2, then you may score mitotic count as follows:[15]
- 1 point: ≤7 mitoses per 10 HPFs
- 2 points: 8 to 14 mitoses per 10 HPFs
- 3 points: ≥15 mitoses per 10 HPFs
If you have a significant different HPF area or you are not sure, count 10 HPFs and calculate the number of mitoses per mm2 (Further information: Evaluation#Counts per mm2 ):
- 1 point: ≤3 mitoses per mm2
- 2 points: 4 to 7 mitoses per mm2
- 3 points: ≥7 mitoses per mm2
A table of counts for various HPF sizes is available at the College of American Pathologists. [1] (Page 22)
Overall grade
The scores for each of these three criteria are added together to give a final overall score and a corresponding grade as follows:
- 3-5 Grade 1 tumor (well-differentiated). Best prognosis.
- 6-7 Grade 2 tumor (moderately differentiated). Medium prognosis.
- 8-9 Grade 3 tumor (poorly differentiated). Worst prognosis.
Microcalcifications

If invasive ductal carcinoma is seen, make at least a low power screening for microcalcifications (to correlate with imaging), but there's no need to look carefully (as tiny microcalcifications would unlikely correlate with imaging anyways).
Immunohistochemistry
Look at local protocols for what immunohistochemistry tests and other biomarker test need to be tested for each case of invasive breast cancer, or what needs to be retested for subsequent excisions at the primary site or at metastatic sites.[note 3]
Ki-67 index
Ki-67 index is mainly relevant in those with stage T1-T2, N0-N1, to determine if chemotherapy is needed (if Ki67 is >30% rather than <5%).[16]
Ki-67 index is most feasibly quantified by a hot spot method,[note 4] Hot spots are areas in which Ki-67 staining is particularly higher relative to the adjacent tumor areas.[17] Usually, the invasive edge of a tumor is a hot spot.[17] When a tumor had several hot spots, the “hottest” spot is selected.[17] Aim to count at least 500 cells in each case, but this is not always possible in cases with low tumor cell density and small tumor size.[17] Also aim to include at least three high-power (×40 objective) fields. Count a nucleus as “positive” if there is any definite brown staining in the nucleus of an invasive breast cancer cell, above the surrounding background in the cytoplasm and extracellular matrix.[18] If a comparisons must be made between core biopsies and sections from an excision, evaluation of the latter should be across the whole tumor.[16] Only nuclear staining counts. Staining intensity of a positive nucleus is not relevant.[16]
HER2
HER2 can initially be evaluated by immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH). If IHC is performed first and is borderline/equivocal, then FISH is recommended.[19] If FISH is performed first and indicates that further workup is required, then IHC may be the performed as per established algorithms.[note 5]
HER2 immunohistochemistry
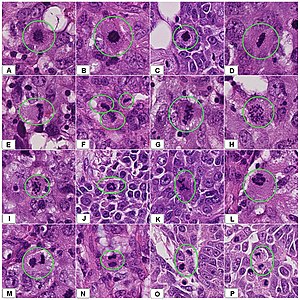
Look at different parts of the tumor, and evaluate the area(s) with most staining. When negative of faint staining is seen, evaluate at high magnification.
| Score[20][21] | Pattern[22] | Status[20][21] |
|---|---|---|
| 0 | Either:[22]
|
HER2 negative (not present) |
| 1+ | Incomplete membrane staining that is faint or barely perceptible and within >10% of the invasive tumor cells.[22] | |
| 2+ | Weak to moderate complete membrane staining observed in >10% of tumor cells.[22] | Borderline/Equivocal |
| 3+ | Circumferential membrane staining that is complete, intense, and in >10% of tumor cells.[22] | HER2 positive |
Micrographs showing each score:[23]
HER2 FISH
HER2 FISH usually uses chromosome enumeration probe 17 (CEP17) to count the amount of chromosomes. Hence, the HER2/CEP17 ratio reflects any amplification of HER2 as compared to the number of chromosomes.
To prepare a slide for HER2 testing, you may need to choose a paraffin-embedded and mark the resulting slide so that you or whoever interprets it knows where to look for the target tumor cells. When there are multiple blocks of the same case, choose the the one with most tumor. (If a block has undergone sectioning for immunohistochemistry (such as ER, PR and/or Ki67) make sure that you have a new H&E slide at a level next to the one to be used for FISH, so that they will correlate better.) In cases of both invasive and in situ carcinoma in the same specimen, mark all invasive carcinoma (also for crushed tissue or with other artifacts) but not the in situ carcinoma. Also mark a small area of normal tissue as an internal control. If possible, it should be a bit away from the tumor, even if only consisting of fatty tissue.
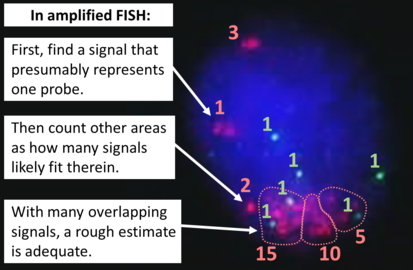
To interpret a HER2 FISH study, first perform a quality control check of the slide as per manufacturer and/or local protocol (generally including checking for proper signals from a control specimen). In cases of both invasive and in situ carcinoma in the same specimen, only score the invasive cells. The signals of 20 cells are usually counted. Also focus up and down on each nucleus to find all signals therein.
If a cytotechnologist has already performed a count, you do not have to recount, but make sure the count is reasonable regarding what you see. In any case, also look around for any obvious tumor heterogeneity in HER2 signals.
If the HER2/CEP17 ratio is borderline (1.8-2.2), count an additional 20 nuclei and recalculate a ratio for the total of 40 nuclei.
| HER2/CEP17 ratio | |||
|---|---|---|---|
| ≥2.0 | <2.0 | ||
| Average HER2 copy number per cell | ≥4.0 | HER2 positive | Additional work-up required[note 5] |
| <4.0 | Additional work-up required[note 5] | HER2 negative | |
If the initial HER2 result is negative for a needle biopsy of a primary breast cancer, a new HER2 test may be performed on the subsequent breast excision.[note 5]
Estrogen and progesterone receptors
Generally perform immunohistochemistry for estrogen and progesterone receptors and calculate the percentage of positive tumor cells.
Neoadjuvant cases

If not already stated, look for clues that neoadjuvant treatment has been given, that is, when the patient has received chemotherapy, endocrine therapy and/or radiotherapy before the excision. Look more thoroughly for it if the duration between a previous biopsy and subsequent excision is longer than usual. It's usually about a month.[25]
In neoadjuvant cases , perform:
- Measurement of the tumor bed which generally manifests as a fibroelastic area.
- Classification of residual cancer, for which there are multiple systems, mainly Residual Cancer Burden (RCB)[note 6] and the American Joint Committee on Cancer post-neoadjuvant therapy staging system (yAJCC).[26]
- Further information: Evaluation of tumors
Report
Breast excision
- Tumor size, if not already given from gross report.[3] Give 3 dimensions or greatest dimension.[3]
- Histopathologic subtype if apparent, but "invasive carcinoma" is acceptable.
- Stage[3]
- Grade, preferably by overall BRE grade. Optionally, give scores for the components thereof.[3]
- Extent of any angiolymphatic invasion.[3]
- Margins of resection,[3] as closest distance from carcinoma to margin in mm or cm or "tumor on ink"/"carcinoma is present on margin". ((If applicable, also specify as "close margins" (no tumor on ink but <2 mm), or "negative margins" (≥2 mm).))[27]
- Results of any immunohistochemistry and other tests[3]
- HER2 as a score or status.
- Ki-67, preferably as labeling index
Example:
| Breast excision with 70 x 55 x 18 mm ductal invasive breast cancer. Nottingham grade II. Estrogen receptor positive, progesterone receptor negative, HER2 receptor score 0, Ki-67 index 17%, T1b. Radically removed. |
Needle or core biopsy
- Histopathologic subtype if apparent, but "invasive carcinoma" is acceptable.
- Results of any immunohistochemistry and other tests, as per excision[3]
- Presence of absence of lymphatic and/or vascular invasion[3]
- Optionally: Provisional grading. Grading can alternatively be deferred to excision.[3]
- State if studies are deferred for a later excision sample[3]
For cancers, generally include a synoptic report, such as per College of American Pathologists (CAP) protocols at cap.org/protocols-and-guidelines.
- synoptic report example
- Tumor type: invasive ductal carcinoma with micropapillary pattern
- Tumor size: greatest microscopic measurement of invasive carcinoma in positive core(s)): 0.7 cm
- In-situ component: no
- Microscopic grading (Nottingham modification of the Bloom-Richardson system):
- Only applies to infiltrating ductal and lobular carcinoma:
- Tubule formation: Little or none (score =3)
- Nuclear pleomorphism: Marked variation in size, nucleoli, chromatin clumping, etc. (score =3)
- Mitotic count : Less than 6 mitoses per 10 hpf (score =1)
- Composite score: 7 points (applies to infiltrating ductal and lobular carcinoma only)
- Histologic grade: Grade II: 6-7 points
- Nuclear grade: grade 3
- Microcalcifications: Present in non-neoplastic tissue
- Lymphocytic host response: absent
- Necrosis: absent
- Blood vessel invasion: absent
- Lymphatic and/or vascular invasion: absent
- Skin involvement: not applicable
- Results of immunohistochemical stains for prognostic markers (as per original report):
- Estrogen Receptor (ER) Status: Positive (greater than 10% of cells demonstrate nuclear positivity)
- Percentage of Cells with Nuclear Positivity: 91-100%
- Average Intensity of Staining: Strong
- Progesterone Receptor (PgR) Status: Positive
- Percentage of Cells with Nuclear Positivity: 51-60%
- Average Intensity of Staining: Strong, moderate and weak
- Estrogen Receptor (ER) Status: Positive (greater than 10% of cells demonstrate nuclear positivity)
- HER-2 by IHC: 2+ / Equivocal
- REFLEX HER-2 FISH TEST: Nonamplificed (ratio 1.5; 3.5 Her-2 signals/cell)
- Ki-67:
- Percentage of Cells with Nuclear Positivity: 43%
- Primary Antibody: MIB1
- Cold Ischemia and Fixation Times: 3 minutes
- Fixation Time (hours): 14 hours and 33 minutes
- Fixative: formalin
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
- ↑ For myoepithelial markers, a combination of p63 and calponin is generally recommended for breast lesions. D2-40 is useful for highlighting lymphatics for invasion.
- ↑ If the previous biopsy was negative for ER and PR receptors, and the patient has undergone neoadjuvant chemotherapy before excision, then generally retest ER/PR on the excision. Retesting ER/PR on any excision with previously negative ER/PR on biopsy on a patient having received neoadjuvant therapy has no scientific support nor opposition.
- William M Sikov, MD, FACP, FNCBCJudy C Boughey, MD, FACSZahraa Al-Hilli, MD, FACS, FRCSI. General principles of neoadjuvant management of breast cancer. UpToDate. In breast cancer metastases, generally retest estrogen and progesterone receptors, and HER2 in the following circumstances:- If the status of the primary tumor is unknown or negative for ER/PR and/or HER2
- If the primary tumor is heterogeneous for ER/PR expression
- If the metastatic progression is unusual for the tumor characteristics
- If the relapse is unexpectedly early or late
- If unusual metastasis location
- If the initial test was performed more than 10 years ago
- If the testing turnaround time are relatively short (to reduce potential delays in patient management by retesting)
- ↑ Besides from a hot spot method of Ki67 counting, there is also a IKWG global average method which is more comprehensive. However, the inter-observer difference between the hot spot method and the 'IKWG global average is not statistically significant, and has not shown any significant difference in clinical outcome (theoretically, the area of highest Ki-67 proliferative index is probably most likely to correlate with malignant transformation and risk of metastasis, making the hot spot both more straightforward and clinically relevant than a global average).
- Reference and instructions for the IKWG global average method: Dowsett, M.; Nielsen, T. O.; A'Hern, R.; Bartlett, J.; Coombes, R. C.; Cuzick, J.; Ellis, M.; Henry, N. L.; et al. (2011). "Assessment of Ki67 in Breast Cancer: Recommendations from the International Ki67 in Breast Cancer Working Group ". JNCI Journal of the National Cancer Institute 103 (22): 1656–1664. doi:. ISSN 0027-8874. - ↑ 5.0 5.1 5.2 5.3 5.4 5.5 If additional work-up is required by FISH study, see source article for detailed algorithms:
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS (2018). "Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. ". J Clin Oncol 36 (20): 2105-2122. doi:. PMID 29846122. Archived from the original. . - ↑ A calculator and explanations for calculating RCB is found at: http://www3.mdanderson.org/app/medcalc/index.cfm?pagename=jsconvert3
Main page
References
- ↑ 1.0 1.1 Monika Roychowdhury. Breast - Invasive breast carcinoma of no special type and variants - NST (ductal). Pathology Outlines. Topic Completed: 1 September 2009. Minor changes: 17 September 2020
- ↑ Hurd TC, Sneige N, Allen PK, Strom EA, McNeese MD, Babiera GV (1997). "Impact of extensive intraductal component on recurrence and survival in patients with stage I or II breast cancer treated with breast conservation therapy. ". Ann Surg Oncol 4 (2): 119-24. doi:. PMID 9084847. Archived from the original. .
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 3.13 3.14 3.15 . Infiltrating Ductal Carcinoma of the Breast (Carcinoma of No Special Type). Stanford Medical School. Retrieved on 2019-10-02.
- ↑ 4.0 4.1 4.2 Originally copied from Fadi M. Alkabban; Troy Ferguson. Cancer, Breast. National Center for Biotechnology Information. Last Update: June 4, 2019. Creative Commons Attribution 4.0 International License
- ↑ "Internal mammary lymphadenopathy in breast carcinoma: CT appraisal of anatomic distribution ". Radiology 167 (1): 89–91. April 1988. doi:. PMID 3347753.
- ↑ "Internal mammary lymphadenopathy: imaging of a vital lymphatic pathway in breast cancer ". Radiographics 10 (5): 857–70. September 1990. doi:. PMID 2217975.
- ↑ Elston, CW; Ellis, IO (1991). "Pathologic prognostic factors in breast cancer. I. The value of histological grades in breast cancer. Experience from a large study with long-term follow-up ". Histopathology 19 (5): 403–10. doi:. PMID 1757079. "Republished ". Histopathology 41: 154–161. 2002. doi:.
- ↑ Oudai Hassan. What is the Nottingham combined histologic grade (modified Scarff-Bloom-Richardson grade) system for breast tumors?. Medscape. Updated: Mar 20, 2019
- ↑ Al-Kuraya, Khawla; Schraml, Peter (2004). "Prognostic relevance of gene amplifications and coamplifications in breast cancer ". Cancer Research 64 (23): 8534–8540.
- ↑ Elston CW, Ellis IO. Pathologic prognostic factors in breast cancer. I. The value of histological grades in breast cancer. Experience from a large study with long-term follow-up. Histopathology 1991, 19:403-410.
- ↑ Bloom, H.J.; Richardson, W.W. (1957). "Histological grading and prognosis in breast cancer; A study of 1409 cases of which 359 have been followed for 15 years ". British Journal of Cancer 11 (3): 359–77. doi:. PMID 13499785.
- ↑ Genestie, C.; Zafrani, B.; Asselain, B.; Fourquet, A.; Rozan, S.; Validire, P.; Vincent-Salomon, A.; Sastre-Garau, X. (1998). "Comparison of the prognostic value of Scarff-Bloom-Richardson and Nottingham histological grades in a series of 825 cases of breast cancer: Major importance of the mitotic count as a component of both grading systems ". Anticancer Research 18 (1B): 571–6. PMID 9568179.
- ↑ Pujani, Mukta; Sharma, KiranLata; Srivastava, AN; Singh, US; Bansal, Cherry (2014). "Grading systems in the cytological diagnosis of breast cancer: A review ". Journal of Cancer Research and Therapeutics 10 (4): 839. doi:. ISSN 0973-1482.
- ↑ van Dooijeweert C, van Diest PJ, Ellis IO (2022). "Grading of invasive breast carcinoma: the way forward. ". Virchows Arch 480 (1): 33-43. doi:. PMID 34196797. PMC: 8983621. Archived from the original. .
- ↑ Error on call to Template:cite web: Parameters url and title must be specified. . College of American Pathologists. Protocol Posting Date: September 2022
- ↑ 16.0 16.1 16.2 Dowsett, M.; Nielsen, T. O.; A'Hern, R.; Bartlett, J.; Coombes, R. C.; Cuzick, J.; Ellis, M.; Henry, N. L.; et al. (2011). "Assessment of Ki67 in Breast Cancer: Recommendations from the International Ki67 in Breast Cancer Working Group ". JNCI Journal of the National Cancer Institute 103 (22): 1656–1664. doi:. ISSN 0027-8874.
- ↑ 17.0 17.1 17.2 17.3 Coleman, William B.; Jang, Min Hye; Kim, Hyun Jung; Chung, Yul Ri; Lee, Yangkyu; Park, So Yeon (2017). "A comparison of Ki-67 counting methods in luminal Breast Cancer: The Average Method vs. the Hot Spot Method ". PLOS ONE 12 (2): e0172031. doi:. ISSN 1932-6203.
- ↑ . Ki67-QC international working group: whole section scoring protocol (global method). International Ki67 in Breast Cancer Working Group (2018-11-29).
- ↑ . Breast Cancer HER2 Status. American Cancer Society. Last Revised: August 25, 2022
- ↑ 20.0 20.1 . IHC Tests (ImmunoHistoChemistry). Breastcancer.org. Last modified on October 23, 2015
- ↑ 21.0 21.1 "Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications ". Molecular Biology International 2014: 852748. 2014. doi:. PMID 25276427.
- ↑ 22.0 22.1 22.2 22.3 22.4 2018 ASCO/CAP guidelines:
- . Figure 1. Algorithm for evaluation of human epidermal growth factor receptor 2 (HER2) protein expression by immunohistochemistry (IHC) assay of the invasive component of a breast cancer specimen.. College of American Pathologists: Homepage. Retrieved on 2022-09-12.
- Ahn S, Woo JW, Lee K, Park SY (2020). "HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation. ". J Pathol Transl Med 54 (1): 34-44. doi:. PMID 31693827. PMC: 6986968. Archived from the original. . - ↑ Nitta H, Kelly BD, Padilla M, Wick N, Brunhoeber P, Bai I (2012). "A gene-protein assay for human epidermal growth factor receptor 2 (HER2): brightfield tricolor visualization of HER2 protein, the HER2 gene, and chromosome 17 centromere (CEN17) in formalin-fixed, paraffin-embedded breast cancer tissue sections.
". Diagn Pathol 7: 60. doi:. PMID 22647525. PMC: 3487810. Archived from the original. .
- "This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0)" - ↑ Diagram and table by Mikael Häggström, MD. Adapted from: Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS (2018). "Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. ". J Clin Oncol 36 (20): 2105-2122. doi:. PMID 29846122. Archived from the original. .
- ↑ van Esser S, Peters NH, van den Bosch MA, Mali WP, Peeters PH, Borel Rinkes IH (2009). "Surgical outcome of patients with core-biopsy-proven nonpalpable breast carcinoma: a large cohort follow-up study. ". Ann Surg Oncol 16 (8): 2252-8. doi:. PMID 19437077. PMC: 2711911. Archived from the original. .
- ↑ Campbell JI, Yau C, Krass P, Moore D, Carey LA, Au A (2017). "Comparison of residual cancer burden, American Joint Committee on Cancer staging and pathologic complete response in breast cancer after neoadjuvant chemotherapy: results from the I-SPY 1 TRIAL (CALGB 150007/150012; ACRIN 6657). ". Breast Cancer Res Treat 165 (1): 181-191. doi:. PMID 28577078. PMC: 5653207. Archived from the original. .
- ↑ 27.0 27.1 Bundred JR, Michael S, Stuart B, Cutress RI, Beckmann K, Holleczek B (2022). "Margin status and survival outcomes after breast cancer conservation surgery: prospectively registered systematic review and meta-analysis. ". BMJ 378: e070346. doi:. PMID 36130770. PMC: 9490551. Archived from the original. .
Image sources
- ↑ 1.0 1.1 Image(s) by: Mikael Häggström, M.D. Public Domain
- Author info
- Reusing images