Other surgical pathology
Contents
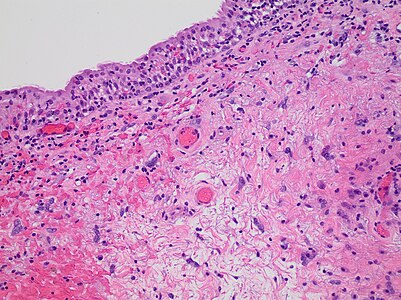
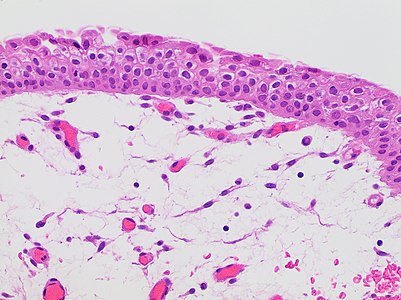
Cornea
Author:
Mikael Häggström [note 1]
Comprehensiveness
On this resource, the following formatting is used for comprehensiveness:
- Minimal depth
- (Moderate depth)
- ((Comprehensive))
Gross processing
- Inspect
- Measure
- Serially section in 3-4 mm wide slices.
Example gross report:
| (Labeled: ___. The specimen is received in formalin and consists of an) << opaque / translucent>> corneal disc measuring __ cm in diameter and __ cm in thickness. The specimen is serially sectioned and entirely submitted for microscopic examination in one cassette. |
Microscopic examination
Look for integrity of Bowman's and Descemet's membranes.
For corneal opacities, look for the most common causes, which generally manifest as:[1]
- Inflammation and edema
- Traumatic injury
Reporting
Example:
|
(Right eye cornea, excision: |
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
Main page
References
- ↑ Michael Woods (2018). Corneal Opacity. Winchester Hospital, MA.
Image sources
Urinary bladder
Author:
Mikael Häggström [note 1]
Urinary bladder biopsy
Usually performed as a transuretral resection of the bladder (TURB).
Gross reporting of transurethral resections
- Generally submit all material. (It may be sufficient to submit representative sections that include the muscular layer, if grossly identified. Yet, many departments require submission of the entire specimen regardless, so if unsure, that is the safe choice.)
- If transported or processed together with other cases, put the samples in thin-mesh cassettes or tissue bags to limit contamination.[note 2]
Example report:
| Container A. Labeled "bladder tumor". The specimen is received in formalin and consists of multiple fragments of tan-gray, friable soft tissue measuring about __ x __ x __ cm in aggregate. The specimen is entirely submitted for microscopic examination in __ cassettes. |
Microscopy
Mainly look for urothelial carcinoma (also called transitional cell carcinoma), which constitutes 95% of bladder cancers.[1]
Low grade urothelial carcinoma: Urothelium is thickened but only slightly atypical and has maintained polarity.
High grade urothelial carcinoma: Loss of polarity and severe abnormal cytology.
In contrast, an inverted urothelial papilloma has smooth surface with minimal to absent exophytic component, is well circumscribed with smooth base, and has no obvious infiltration and no/minimal cytologic atypia.[2]
Other possibilities:
Squamous cell carcinoma of the urinary bladder. Further information: Urothelial versus squamous cell carcinoma
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
- ↑ Urothelial carcinoma is a promiscuous contaminant of other tissues.
- Carll T, Fuja C, Antic T, Lastra R, Pytel P (2022). "Tissue Contamination During Transportation of Formalin-Fixed, Paraffin-Embedded Blocks. ". Am J Clin Pathol 158 (1): 96-104. doi:. PMID 35195717. Archived from the original. .
Main page
References
- ↑ . Types of Bladder Cancer: TCC & Other Variants. CTCA.
- ↑ Monika Roychowdhury. Bladder, ureter & renal pelvis - Urothelial neoplasms - noninvasive - Inverted urothelial papilloma. Pathology Outlines. Topic Completed: 1 December 2014. Minor changes: 3 December 2020
Image sources
Urine cytology
Author:
Mikael Häggström [note 1]
Clinical information
It is not necessary to look through more than readily available reports from previous urine cytologies.
Evaluation
Mainly look for:
Reactive urothelial changes, Pap stain, showing urothelial cells with enlarged nuclei but a nucleus-cytoplasm ratio of less than 0.5. There are bacteria, as well as an inflammatory response of neutrophils, providing a cause for the changes. Can be reported as "Benign urothelial cells, neutrophils and bacteria".
High-grade urothelial carcinoma. Cytologic diagnosis of high-grade urothelial carcinoma requires > 10 cells with high N/C ratio, irregular chromatin pattern and hyperchromatic nuclei (Pap stain).[1]

The N/C ratios apply to the finding of any cells meeting the criteria, and not the average among atypical cells (in the majority of obviously positive cases, N/C/ratio averages 0.5).[2] When there are obvious features of malignancy, there is no need to hunt for cells that fulfil all criteria to make such diagnosis.[2]
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
Main page
References
- ↑ Wang Y, Auger M, Kanber Y, Caglar D, Brimo F (2018). "Implementing The Paris System for Reporting Urinary Cytology results in a decrease in the rate of the "atypical" category and an increase in its prediction of subsequent high-grade urothelial carcinoma. ". Cancer Cytopathol 126 (3): 207-214. doi:. PMID 29278461. Archived from the original. .
- ↑ 2.0 2.1 2.2 - Image by Mikael Häggström. Reference: Wojcik EM, Kurtycz DFI, Rosenthal DL (2022). "We'll always have Paris The Paris System for Reporting Urinary Cytology 2022. ". J Am Soc Cytopathol 11 (2): 62-66. doi:. PMID 35094954. Archived from the original. .
Image sources
Digit
Author:
Mikael Häggström [note 1]
For an amputated toe or finger:
Comprehensiveness
On this resource, the following formatting is used for comprehensiveness:
- Minimal depth
- (Moderate depth)
- ((Comprehensive))
- Other legend
<< Decision needed between alternatives separated by / signs >>
{{Common findings / In case of findings}}
[[Comments]]
Link to another page
Intitial processing
- Measure length and average diameter
- Determine the anatomic location of the cut (proximal to the distal phalanx, middle phalanx, metatarsal phalangeal joint etc).
- Look at soft tissue margins whether they look viable or necrotic.
- Look at the skin surface. For any lesion such as ulcerated or necrotic one, measure the distance to the nearest soft tissue margin.
- Ink the surgical margins differently for soft tissue and bony margin(, including if the bony margin is a joint surface ("cartilaginous margin").)
- Split the digit longitudinally, either in the midline or at the closest margin between any ulcer and the soft tissue margin.
- Let the tissue fix in formalin and then use a relatively strong decalcifying agent, usually at least 5-6 hours.
Tissue selection
Sections for microscopy are taken as follows:
For amputations disarticulated at the joint:
- Perpendicular sections of the articular cartilage and adjacent bone. State which bone in key of sections.
For amputations resected by cutting across bone:
- Ink the bone at the proximal margin, submit perpendicular section of bony margin.
Gangrene and/or suspected osteomyelitis:
- Submit cross sections of each bone with associated ulcer and/or gangrene if appropriate, generally sagittal/longitudinal. Attempt to include the longitudinal distance from potential osteomyelitis to the proximal bony/cartilaginous margin.
- Skin and soft tissues at proximal margin.
- If margin is close to gangrene: perpendicular sections
- If far, submit en face[note 2]
- If you receive a separate thin or discoid bone slice, it is generally an additional bone margin. Preferably, have this decalcified and processed promptly, so that it may be used to make a preliminary report as to whether it is involved even before the main specimen is processed.
At least if osteomyelitis is suspected, ink all proximal (and/)or distal cut surfaces (differentially) of each slice, so that the pieces can be oriented, and the distance between osteomyelitis and the surgical margin can be estimated. The exception is where pieces can most definitely be anatomically oriented, such as the presence of a nail distally.
Gross report
Example:
| (A. Labeled - ___. The specimen is received in formalin and consists of an amputated toe/finger.) The digit measures ___ cm in length and ___ cm in average diameter. The digit is resected ___ [[location]]. {{The proximal ___ cm of the specimen is not covered by skin and soft tissue.}} The skin and soft tissue margins appear <viable / necrotic>. The skin surface of the digit appears ___ {{and displays an (ulcerated/necrotic/gangrenous) lesion, cm from the cutaneous margin}}. The nail is <color/thickened/absent/necrotic>. The soft tissue surgical margin is inked blue [[for example]], and the <<bony surgical / cartilaginous>> margin of the ___ [[specific bone involved]] is inked green [[for example]]. On cut sections, the bone subjacent to the ulcer shows no gross abnormalities. Representative sections are submitted for microscopic examination in ___ cassettes following decalcification. Key to sections:
|
Microscopic examination
Mainly, detect the presence of:
Osteomyelitis, mainly by neutrophils in the stroma. Further information: Osteomyelitis
- Microscopic report
Example:
| (A. Left third toe, amputation:) Toe with ulcer, gangrene and osteomyelitis. Osteomyelitis involves the distal phalanx, middle phalanx and proximal phalanx. Osteomyelitis is 2.0 cm from the proximal articular surface of the proximal phalanx. (The skin and soft tissue at the surgical margin appear viable.) |
Bone
Grossing
To submit slides for microscopy, generally gross as follows:
- Split the bone in the plane of interest for microscopy slides.
- Fix the bone in formalin.
- Perform decalcification of the specimen. First, generally take at least a small piece to be kept separately in formalin, in case the main specimen becomes necrotic, so that you have at least one more chance to decalcify it more lightly. If the order and/or history is suspicious for metastasis, try to sample a part of the specimen that is soft enough to not need decalcification (to avoid the risk that decalcification will impair later immunohistochemistry or other testing).
- Take the sections of interest.
Microscopic evaluation
Look for any of the following:
Acute osteomyelitis: Numerous neutrophils in the stroma.
Metastasis
Highly suspicious prostate adenocarcinoma metastasis can be confirmed with NKX1, TTF1 and CDX2.
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
- ↑ En face means that the section is tangential to the region of interest (such as a lesion) of a specimen. Further information: Gross_processing#Cutting
Main page
References
Image sources
Pituitary
Author:
Mikael Häggström [note 1]
Microscopic evaluation
- Look for cellular expansions, which in the anterior pituitary confers a loss of normal cellular heterogeneity.
- If present, distinguish it as hyperplasia or neoplasia. If uncertain, by using reticulin immunohistochemistry, hyperplasia will show a preserved reticulin meshwork, whereas pituitary adenomas have disruption of it.[1]
- For adenomas, unless otherwise indicated by the medical history, will generally by definition be of the silent type. Basophilic or acidophilic staining may give a clue about the subtype, but immunohistochemistry is generally required.
Incidences of silent pituitary adenomas.[2]
A silent gonadotroph pituitary adenoma which is in this case is eosinophilic (contrary to normal, basophilic, gonadotroph cells).[3]
Microscopy report
In normal autopsy:
| Adenohypophysis and neurohypophysis with no focal changes. |
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
Main page
References
- ↑ Al-Brahim, N Y Y; Asa, S L (2006). "My approach to pathology of the pituitary gland ". Journal of Clinical Pathology 59 (12): 1245–1253. doi:. ISSN 0021-9746.
- ↑ Drummond, Juliana; Roncaroli, Federico; Grossman, Ashley B; Korbonits, Márta (2019). "Clinical and Pathological Aspects of Silent Pituitary Adenomas
". The Journal of Clinical Endocrinology & Metabolism 104 (7): 2473–2489. doi:. ISSN 0021-972X.
- "This article has been published under the terms of the Creative Commons Attribution License (CC BY; https://creativecommons.org/licenses/by/4.0/)" - ↑ Gaballa, Salem; Lindsay, Jane; AlJaf, Avan; Hlaing, Kyaw M; Patel, Kashyap (2020). "Acute Unilateral Oculomotor Nerve Palsy as the Initial Presenting Sign of Nonfunctioning Apoplectic Gonadotroph Adenoma
". Cureus. doi:. ISSN 2168-8184.
- "This is an open access article distributed under the terms of the Creative Commons Attribution License CC-BY 4.0"
Image sources
Brain/meningeal tumor
Intraoperative consultation of brain tumor fragments
Preparation
If you are expecting a brain/meningeal tumor, look at any radiology to find out what is the suspected diagnosis or differential diagnoses. A connection to the dura raises the suspicion of a meningioma. Multiple tumors raises the suspicion of metastasis or lymphoma.
Grossing
Measure the size of the specimen in 3 dimensions.

- A woven architectural pattern
- Psammoma bodies (spheroid calcifications)
- Syncytial cells (having indistinct cell membranes) with eosinophilic (pink) cytoplasms
- Round uniform nuclei
- Whorls (concentric cell arrangements)[1]
Squash prep
Remove a drop-size sample, place it on a glass-slide, then gently smear it out with another glass slide, followed by applying a fixative solution and staining with H&E.
Evaluation
The most common primary brain tumors are:[2]
- Gliomas[3] (50.4%)
- Meningiomas[3] (20.8%)
- Pituitary adenomas[3] (15%)
- Nerve sheath tumors (10%)
Also look into the patient's history for past cancers that may have metastasized to the brain.
The location, younger vs older age, as well as the overall pattern provide the main differential diagnoses:
Location and younger vs older age
| Location | Child or young adult | Older adult |
|---|---|---|
| Cerebral, supratentorial | Ganglioglioma, dysembryoplastic neuroepithelial tumor (DNET), pleomorphic xanthoastrocytoma (PXA),
ependymoma, atypical teratoid/rhabdoid tumor (AT/RT), CNS embryonal neoplasms |
Glioblastoma, infiltrating astrocytoma (grades II-III), oligodendroglioma, metastasis, lymphoma, infection |
| Cerebellar, infratentorial, fourth ventricle | Pilocytic astrocytoma, medulloblastoma, ependymoma, choroid plexus papilloma, atypical teratoid/rhabdoid tumor (AT/RT) | Metastasis, hemangioblastoma, choroid plexus papilloma, subependymoma |
| Brainstem | Pilocytic astrocytoma, diffuse midline glioma | Astrocytoma, glioblastoma, diffuse midline glioma, metastasis |
| Spinal cord (intramedullary) | Ependymoma, pilocytic astrocytoma, diffuse midline glioma, myxopapillary ependymoma, drop metastasis | Ependymoma, astrocytoma, diffuse midline glioma, myxopapillary ependymoma (filum terminale), paraganglioma (filum terminale) |
| Spinal cord (extramedullary) | Meningioma, schwannoma, metastasis, melanocytoma, melanoma | Schwannoma, meningioma, melanocytoma, melanoma, malignant peripheral nerve sheath tumor (MPNST) |
| Spinal cord (extradural) | Bone tumor, meningioma, abscess, vascular malformation, | Herniated disk, lymphoma, abscess, metastases, |
| Extra-axial, dural, leptomeningeal | Leukemia/lymphoma, Ewing sarcoma, rhabdomyosarcoma, disseminated medulloblastoma, diffuse leptomeningeal glioneuronal tumor (DLGNT), | Meningioma, solitary fibrous tumor, metastasis, lymphoma |
| Sellar/infundibular | Pituitary adenoma, craniopharyngioma, Rathke cleft cyst, pituicytoma, Langerhans cell histiocytosis (LCH), germ cell tumors | Pituitary adenoma, craniopharyngioma, Rathke cleft cyst, pituicytoma, meningioma,
metastasis, chordoma |
| Suprasellar, hypothalamic, optic pathway, third ventricle | Germ cell tumors, craniopharyngioma, pituitary adenoma, optic glioma, Langerhans cell histiocytosis (LCH) | Colloid cyst, craniopharyngioma, chordoid glioma |
| Pineal | Germ cell tumors, pineocytoma, pineoblastoma, pineal cyst | Pineocytoma, pineal cyst, pineal parenchymal tumors of intermediate differentiation (PPTID) |
| Thalamus | Pituitary adenoma, diffuse midline glioma | Diffuse midline glioma, glioblastoma, lymphoma |
| Lateral ventricle | Central neurocytoma, subependymal giant cell astrocytoma (SEGA), choroid plexus papilloma/carcinoma,
meningioma |
Central neurocytoma, subependymal giant cell astrocytoma (SEGA), choroid plexus papilloma/carcinoma, subependymoma, meningioma |
| Nerve root, paraspinal | Neurofibroma, schwannoma, malignant peripheral nerve sheath tumor (MPNST) | Neurofibroma, schwannoma, MPNST, lymphoma, meningioma |
| Cerebellopontine angle | Schwannoma, Choroid plexus papilloma, atypical teratoid/rhabdoid tumor (AT/RT) | Schwannoma, meningioma, epidermoid cyst, choroid plexus papilloma, endolymphatic sac tumor |
Overall patterns
Hypercellular infiltrate but intact architecture:
A sharply demarcated lesion:
Mostly solid, but with ill-defined margin towards adjacent brain tissue:
Extensive necrosis and destruction of normal tissue:
|
Centered around blood vessels:
Outside brain or spine
Only very subtle abnormalities:
Subarachnoid space expansion:
|
Meningioma
Microscopic evaluation

- A woven architectural pattern
- Psammoma bodies (spheroid calcifications)
- Syncytial cells (having indistinct cell membranes) with eosinophilic (pink) cytoplasms
- Round uniform nuclei
- Whorls (concentric cell arrangements)[5]
Look for the signs of both typical meningioma and main histological variants. On frozen sections, mention the presence of atypia/high-grade cells.
On permanent sections, classify by WHO grade:
| WHO Grade I | WHO Grade II | WHO Grade III |
|---|---|---|
| Benign | Atypical | Malignant |
|
|
|
Reporting
Example frozen section report:
| A. Right frontal tumor: -Meningioma. Negative for atypia. |
Example permanent section report:
| A. Right frontal tumor: -Meningioma, WHO grade 1. |
- ↑ Image by Mikael Häggström, MD. Reference for typical findings: Chunyu Cai, M.D., Ph.D.. Meningioma. Pathology Outlines. Last author update: 10 November 2021}}
- ↑ Park, Bong Jin; Kim, Han Kyu; Sade, Burak; Lee, Joung H. (2009). "Epidemiology". Meningiomas: Diagnosis, Treatment, and Outcome . Springer. p. 11. ISBN 978-1-84882-910-7.
- ↑ 3.0 3.1 3.2 . Brain Tumors - Classifications, Symptoms, Diagnosis and Treatments (in en). www.aans.org.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 From notes by Dr. Kurt Schaberg, in turn citing: Perry, Arie; Brat, Daniel J. (2017-12-07). Practical Surgical Neuropathology . Philadelphia, PA: Churchill Livingstone. ISBN 978-0-323-44941-0.
- ↑ Image by Mikael Häggström, MD. Reference for typical findings: Chunyu Cai, M.D., Ph.D.. Meningioma. Pathology Outlines. Last author update: 10 November 2021}}
- ↑ Brabec J, Friedjungová M, Vašata D, Englund E, Bengzon J, Knutsson L (2023). "Meningioma microstructure assessed by diffusion MRI: An investigation of the source of mean diffusivity and fractional anisotropy by quantitative histology.
". Neuroimage Clin 37: 103365. doi:. PMID 36898293. PMC: 10020119. Archived from the original. .
- "This is an open access article under the CC BY license (http://creativecommons.org/licenses/by/4.0/)" - ↑ Wu C, Zhong J, Lin L, Chen Y, Xue Y, Shi P (2022). "Segmentation of HE-stained meningioma pathological images based on pseudo-labels.
". PLoS One 17 (2): e0263006. doi:. PMID 35120175. PMC: 8815980. Archived from the original. .
- Creative Commons Attribution 4.0 International license - ↑ Jeffrey I. Traylor, MD and John S. Kuo, MD, PhD, FAANS.. Meningiomas. American Association of Neurological Surgeons. Retrieved on 2024-01-11.