Breast pathology
Author:
Mikael Häggström [note 1]
Contents
- 1 Breast biopsy or excision
- 2 Fibrocystic breast changes
- 3 Fibroepithelial tumor
- 4 Atypical ductal hyperplasia
- 5 Invasive ductal carcinoma
- 6 Invasive lobular carcinoma
- 7 Lobular carcinoma in situ
Breast biopsy or excision
Fresh breast specimens should be put in formalin within one hour.[note 2] Breast specimens should be immersed in formalin for 6-72 hours.[1]
Gross processing
Selection and trimming
For excision (also called lumpectomy):
- Determine total specimen size. ((Weight the specimen[2]))
- Ink margins. If sample orientations are marked, use different colors for different directions.[2]
- Palpate the specimen for masses. If felt, estimate the greatest dimension.[note 3] Compare with radiography if available.[2] Confirm the presence of any known biopsy clips, either visibly or by post-operative radiography (in order to confirm that the specimen includes the region of interest).

- Serially section the specimen.
- When performing triage of fresh lumpectomies, generally make slices 1.1 to 1.5 cm thick. When laid down and flattened, this is generally thin enough to allow for fixation over at least 6 hours, and thick enough to be further cut into 2 or 3 slices after fixation; Note the direction of up/down on each such thick slice so that the relative orientation of the thinner slices thereof is maintained. Slices with solid tumors are cut somewhat thinner since they won't flatten as much when laid down.
- When selecting tissue for submission after fixation, make 3-4 mm thick slices.[2]
- Inspect for any grossly visible lesions. If found, measure at least the greatest dimension of the lesion on the slice where it appears largest. (Also compare the greatest gross measurement (including any palpated one) with the greatest measurement at previous imaging, and note if it confers a staging discrepancy between imaging and gross dimensions, with cutoffs at 0.1 cm, 0.5 cm, 1.0 cm, 2.0 cm, and 5 cm.)
Invasive ductal carcinomas generally appear grossly as tan-white firm areas, here seen (white arrow) on a slice with inked margins. A radioactive seed (black arrow) was inserted by radiology to locate the tumor.
- Re-excisions
Tissue selection

- For lumpectomies, submit:[2]
- Entire specimen if it can fit in 3-5 slices.
- If larger:
- For relatively well demarcated tumors: 1 slice per cm of tumor (minimum of 3 slices of tumor), including both center and periphery of tumor, including closest distance to the surgical margin if possible.
- For diffuse or even invisible tumors such as often is the case for DCIS and ILC, either still submit whole, or use X-ray to guide what tissue to submit.
- Additional suspicious areas, including those indicated by radiography.
- ((Representative sections of margins in each direction.))
Breast specimens where breast cancer is possible should generally not be decalcified even when containing small calcifications, to preserve the ability to perform immunohistochemistry.
See also: General notes on gross processing
Gross report
- Size of original tissue sample, preferably in 3 dimensions.
- Description of inking
- Tumor properties, at least:
- (Description of sectioning and submissions.)
- ((Time of procurement and time of placement in formalin.[note 2]))
Example:
| (The specimen is received fresh and consists of) an irregular fragment of yellow tan fibrofatty tissue measuring 4.0 x 2.8 x 2.0 cm. (The specimen is oriented with two sutures and) the surgical margins are inked as follows: superior-blue, inferior-green, medial-red, lateral-yellow, anterior-orange, and posterior-black. {{A radioactive seed is embedded in the tissue.}} The tissue is serially sectioned {{to reveal a tan-gray, spiculated, indurated mass measuring _cm in greatest dimension. The mass is located _cm from the nearest (<<superior, inferior etc>>) margin. (The specimen is entirely submitted in sequential sections from medial to lateral in 10 cassettes. The medial and lateral margins are submitted as perpendicular sections.) The specimen was procured at _AM/PM on _/_/2020. The specimen was placed in formalin at _AM/PM on _/_/2020. |
Lymph nodes
When lymph nodes are submitted together with a biopsy or excision of a suspected or previously confirmed invasive lobular carcinoma (but not necessarily invasive carcinoma with lobular features), generally put the lymph node specimen through H&E processing at a relative rush. If you don't see any involvement on the H&E stain, order immunostain for CK AE1/AE3 in order to visualize otherwise occult lymph node involvement. The rushing of the lymph node samples allows you to have the immunostained slides by a similar time as the rest of the case.[3]
- Further information: Lymph node
Staining
Usually H&E staining.
Microscopic evaluation
If tumor is found, determine:
- Tumor size
- Malignancy
- Distance from excision margin
Malignancy
The most important is to classify a sample as either of the following:
- Benign
- Carcinoma in situ
- Invasive cancer
Most common types
| Finding | Relative incidence |
Histopathology | Image |
|---|---|---|---|
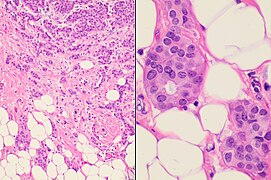
| Fibrocystic breast changes | 40% | Sclerosing adenosis (pictured), with an increase in glandular elements in addition to stromal proliferation that distorts and compresses glands.[5] | 
|
| Radial scar, seen as a fibroelastic stroma and entrapped glands radiating outward. Measure the size of these.[note 4] | 
| ||
| Usual ductal hyperplasia: Cohesive proliferation with haphazard, jumbled cell arrangement or streaming growth pattern. Cells have mild variation in cellular and nuclear size and shape.[6] | 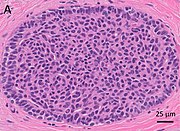
| ||
| No disease | 30% | ||
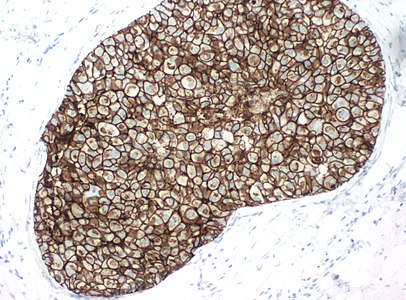
| Fibroepithelial tumor | 7% | Proliferation of both stromal and epithelial components.[note 5] The tumor group mainly includes fibroadenoma and phyllodes tumor. | 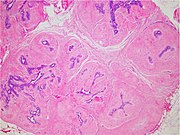
|
| Atypical ductal hyperplasia | 7%[7] | Epithelial proliferations which are not qualitatively or quantitatively abnormal enough to be classified as ductal carcinoma in situ.[7] | 
|
| Other benign mammary dysplasias and neoplasms | 5% | Including:
|

|
| Intraductal papilloma: unremarkable epithelial cells lining fibrovascular cores. | 
| ||
| Pseudoangiomatous stromal hyperplasia (PASH): Complex interanastomosing vessel-like spaces in dense collagenous, keloid-like stroma. | 
| ||
| Breast cancer (in situ or invasive) | 10% | See next section. |
Breast cancer types
| Cancer type | Histopathology | Image |
|---|---|---|
| Invasive ductal carcinoma (IDC) | Carcinomatous cells are seen below the basement membrane of lactiferous ducts. Otherwise, there are no specific histologic characteristics, essentially making it a diagnosis of exclusion.[10] | 
|
| Ductal carcinoma in situ (DCIS) | Malignant epithelial cells confined to the ductal system of the breast, without invasion through the basement membrane.[11] | 
|
| Invasive lobular carcinoma (ILC) | The "classic" pattern is round or ovoid cells with little cytoplasm in a single-file infiltrating pattern, sometimes concentrically giving a targetoid pattern. | 
|
| Lobular carcinoma in situ (LCIS) |
Cells have indistinct cell borders, pale cytoplasm, and uniform small nuclei with evenly distributed chromatin and inconspicuous nucleoli.[12] |

|
| Mucinous carcinoma | Extracellular mucin areas around tumor cells. | 
|
| Medullary carcinoma | Seemingly fused tumor cells (syncytial pattern), and a prominent lymphoid infiltrate. | 
|
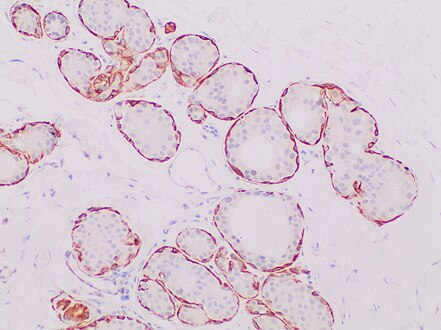
| Solid papillary carcinoma | Larger tumor nests with fibrovascular cores. | 
|
- Further information: Evaluation of tumors
Previous biopsy site
(For excisions or larger, look up past biopsies, and if in the same area, look for biopsy sites in order to confirm that the previous biopsy represents the same pathologies.)
If tumor is not found
Check the imaging indication, and look for abnormalities that may explain the diagnostic findings:
- Dense stromal fibrosis for radiodense areas.
- Calcifications if found on X-ray.
If still not found, note their absence, since it indicates that the biopsy may have missed the target of interest.
Look extra close for invasive lobular carcinoma which may be subtle, as shown
Dense stromal fibrosis, in this case with entrapped fat cells and involuted entrapped ducts (arrows). In contrast, normal breast parenchyma has loose collagenous mammary stroma.[13]
Microscopy report
A normal biopsy may be reported as follows
| (Fibrofatty tissue with benign ducts and lobules.) Negative for (atypia and) malignancy. |
More detailed reports are given at the disease-related articles.
Fibrocystic breast changes
Microscopic examination
Types:
Sclerosing adenosis, with an increase in glandular elements in addition to stromal proliferation that distorts and compresses glands.[15]
Differential diagnosis
Sclerosing adenosis may look similar to invasive ductal carcinoma (IDC), but IDC will:[15]
- Not be lobular at low power
- Have marked cellular atypia
- Have no myoepithelial cells surrounding ducts.
When unsure, perform immunohistochemistry for myoepithelial markers (preferably p63 and calponin in breast lesions):
Fibroepithelial tumor
Gross examination
As per:
or mastectomy.
Microscopic evaluation
Characteristics of fibroepithelial tumor
Biplastic, having proliferation of both stromal and epithelial components,[note 6] arranged into either a pericanalicular pattern (stromal proliferation around epithelial structures), or an intracanalicular pattern (stromal proliferation compressing the epithelial structures into clefts).
Characteristics of fibroadenoma
Fibroadenomas characteristically display hypovascular stroma compared to malignant tumors.[16][17][18] Furthermore, the epithelial proliferation appears in a single terminal ductal unit and has duct-like spaces surrounded by a fibroblastic stroma. The basement membrane is intact.[19]
Characteristics of phyllodes tumor
In contrast to fibroadenomas, phyllodes tumors display a leaf-like architecture, and an increased stromal cellularity.[20] In needle biopsy specimens, phyllodes tumors can be diagnosed in a fibroepithelial tumor if there is prominent mitotic activity of ≥3 per 10 high power fields, or the finding of 3 or more of the following characteristic histologic features:[20]
- Stromal overgrowth
- Fat infiltration
- Stromal fragmentation
- Subepithelial stromal condensation
- Stromal nuclear pleomorphism
Further workup
After diagnosing a fibroepithelial tumor, classify it as benign, borderline or malignant:[21]
| Benign | Borderline | Malignant | |
|---|---|---|---|
| Stromal atypia | Mild | Moderate | Marked |
| Stromal cellularity | Mildly increased, can be focal | Moderately increased, can be focal | Markedly and diffusely increased |
| Stromal overgrowth* | Absent | Absent or very focal | Present |
| Mitotic count | < 5/10 HPF or < 2.5/mm² | 5 - 9/10 HPF or 2.5 - < 5/mm² | ≥ 10/10 HPF or ≥ 5/mm² |
| Tumor border | Well defined | Well defined or focally permeative | Diffusely permeative |
| Malignant heterologous elements | Absent | Absent | Presence directly upgrades to malignant category** |
- * Stromal overgrowth can be defined as absence of epithelial elements containing stroma only in 1 low power field.
- **Malignant categories include chondrosarcoma, liposarcoma (except well differentiated liposarcoma), osteosarcoma, rhabdomyosarcoma, angiosarcoma and leiomyosarcoma
Also, exclude another type of breast cancer, which may arise within it,[22] possibly being:
For borderline and malignant phyllodes tumors, measure the distance from the tumor to the closest surgical margin. However, such measure is not necessary for benign phyllodes tumor.[23]
Microscopic report
If, even after consultation, it is not clear whether a tumor is a fibroadenoma or phyllodes tumor, then it can be reported as a cellular fibroepithelial lesion or a fibroepithelial neoplasm. A benign lesion that resembles but does not clearly show a fibroadenoma can be called a fibroadenomatoid lesion.
A phyllodes tumor warrants a comment whether margins are positive or negative for tumor, whereas fibroadenoma does not need such comment.
Example report of fibroadenoma:
| Right breast, lumpectomy: Fibroadenoma. |
Atypical ductal hyperplasia
Gross examination
As per:
or mastectomy.
Microscopic evaluation

- A - One focus (< 2 mm) of two architecturally disarranged cross sections of tubuli showing a monotonous intraductal proliferation with secondary intraluminal architecture.
- B - One area of an ADH with associated intraluminal calcifications.
- C - Higher magnification of ADH shows low-grade nuclear atypia and monotonous cell proliferation along with secondary intraluminal architecture.
- D - Strong and uniform expression of estrogen receptors (ER). ER immunohistochemistry.
- E - Lack of basal cytokeratins (CK5/6). CK5/6 immunohistochemistry.
Atypical ductal hyperplasia shows epithelial proliferations which are not qualitatively or quantitatively abnormal enough to be classified as ductal carcinoma in situ.[7]
Differential diagnoses
There is no single definite cutoff that separates atypical ductal hyperplasia from ductal carcinoma in situ, but the following are important distinctive features of atypical ductal hyperplasia, with suggested cutoffs:[25]
- Size less than 2 mm.
- Not involving more than one duct.
- The atypical epithelial proliferation is admixed with a second population of proliferative cells without atypia.
- The proliferation completely involves the terminal ductal lobular unit(s), to a limited extent.
Also, ADH tends to have rounded lacunae between cells, in contrast to more crescent-shaped (compressed) lucunae in DCIS.
- Usual ductal hyperplasia (UDH)
In contrast to usual ductal hyperplasia (UDH), atypical ductal hyperplasia (ADH) displays:[6]
- Increased amounts of pale eosinophilic to amphophilic cytoplasm with conspicuous cell borders
- Atypical architectural patterns, including cribriform spaces, Roman arches, trabecular bars and micropapillae
If uncertain, immunohistochemistry can be performed:[6]
| Usual ductal hyperplasia (UDH) | Atypical ductal hyperplasia (ADH) | |
| High molecular weight keratins | Mosaic to occasionally diffuse | Negative |
| Estrogen receptor | Heterogenous staining | Diffusely positive |
Invasive ductal carcinoma
Gross examination
As per:
or mastectomy.
Microscopic evaluation
Malignant epithelial cells confined to the ductal system of the breast.[11] The cells are cohesive and have high grade atypia.[26]
Differential diagnoses
- Invasive ductal carcinoma
- Has invasion through the basement membrane of over 1 mm in size.[27]
DCIS with microinvasion, defined as DCIS with focus of invasive cancer measuring up to 1.0 mm in size.[28]
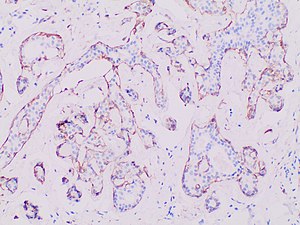
In uncertain cases, use immunohistochemistry stain for calponin (has the highest sensitivity) and p63 (has the highest specificity).
Immunohistochemistry for the myoepithelial marker[note 7] calponin in ductal carcinoma in situ, highlighting myoepithelial cells around all tumor cells, thereby ruling out invasive ductal carcinoma.
Invasive ductal carcinoma with tubular features can look like benign tubules, but calponin and p63 shows no surrounding myoepithelial cells.
There is no single definite cutoff, but the following are suggested cutoffs defining a ductal carcinoma in situ:[25]
- Size over 2 mm.
- Involving more than one duct.
- Lobular carcinoma in situ (LCIS)
Lobular carcinoma in situ (LCIS) displays discohesive cells, often a feathery clear space between cells, solid growth pattern, intracytoplasmic vacuoles, and lack of polarization around luminal spaces.[29]
LCIS typically fills smaller lobules rather than ducts, but DCIS can display lobular cancerization as shown at bottom of image.[image 1]
When unsure, perform immunohistochemistry for E-cadherin and p120. Both E-cadherin (left image below) and p120 (right) have a membranous staining pattern in ductal carcinoma in situ:
In contrast:
Grading
At least a low/intermediate/high grading (by Van Nuys criteria) as follows:[30]
- Low grade DCIS
- Nuclei 10-15 microns (2-3 times the size of a red blood cell)
- Nuclei oval, round, regular, evenly dispersed chromatin up to mildly irregular and minimally pleomorphic
- Nucleoi, if present, are small and indistinct
- Intermediate grade DCIS
- Same nuclear features as low grade

- Substantial tumor cell (comedo) necrosis is present
- High grade DCIS
- Nuclei >15 microns (over 2.5[33] times the size of a red blood cell)
- Nuclei are pleomorphic with clumped chromatin
- Nucleoli are prominent, enlarged
- Necrosis is almost universal and lumenal
(Numerical grading
Use the low/intermediate/high grade to give a numerical grading as follows:[34]
| Feature | Points | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Nuclear grade | Low | Intermediate | High |
| Glands/papillae | >75% | 10% - 75% | <10% |
| Mitotic rate (per 10 HPF) | <1 | 1 - 2 | >2 |
| Central necrosis | <10% | 10% - 50% | >50% |
The points for each feature are added together, giving the following result:[34]
- 4 - 7 points: Grade 1
- 8 - 9 points: Grade 2
- 10 - 12 points: Grade 3
)
Extent
- In biopsies, do not state the extent of DCIS.
- In excisions, state the distance to the closest margin. (Additional extent specification is optional. If there is invasive tumor in addition to DCIS, you may give the DCIS extent in terms of the number of blocks containing DCIS. If an excision has DCIS without any invasive component, consider stating the total size in terms of the greatest distance between any DCIS foci.)
Microcalcifications

If invasive ductal carcinoma is seen, make at least a low power screening for microcalcifications (to correlate with imaging), but there's no need to look carefully (as tiny microcalcifications would unlikely correlate with imaging anyways).
Estrogen and progesterone receptors
Generally perform immunohistochemistry for estrogen and progesterone receptors and calculate the percentage of positive tumor cells.
HER2
((HER2 testing is not necessary, but can be done for prognostic profiling.[35])) See invasive ductal carcinoma for how to evaluate HER2.
Reporting

Example:
| Left breast mass, 2:00, 1 cm from nipple, ultrasound-guided vacuum assisted core needle biopsy: Ductal carcinoma in situ. Negative for invasive carcinoma. |
For cancers, generally include a synoptic report, such as per College of American Pathologists (CAP) protocols at cap.org/protocols-and-guidelines.
See also: General notes on reporting
Invasive lobular carcinoma
Invasive lobular carcinoma (ILC):
Gross examination
As per:
or mastectomy.
Microscopic evaluation
Characteristics
The "classic" pattern is round or ovoid cells with little cytoplasm in a single-file infiltrating pattern, sometimes concentrically giving a targetoid pattern.
Subtyping
The histologic patterns mainly include:[37][38][39]
Differential diagnosis
- In invasive lobular carcinoma, malignant cells have penetrated the basement membrane, in contrast to lobular carcinoma in situ.
- Invasive ductal carcinoma typically has duct-forming tumor cells rather than single files of tumor cells. In uncertain cases, stain for E-cadherin and p120:
Invasive ductal carcinoma, with occasional entrapped normal ducts (arrow).
E-cadherin is negative in invasive lobular carcinoma (shown), but has membranous staining in invasive ductal carcinoma
p120 has cytoplasmic staining in invasive lobular carcinoma (shown), but has membranous staining in invasive ductal carcinoma
Staging
Stage by the TNM system as follows in sections below.
Also, look for any angiolymphatic invasion. If present, check whether it reaches outside the tumor, and if so, how far.[40] Give greatest dimension (,or 3 dimensions, generally by adding up the estimated thicknesses of involved slices)).[40]
Primary Tumor (T)Tumor – Depends on the tumor at the primary site of origin, as follows:[41]
Regional Lymph Nodes (N)Lymph Node: The lymph node values depend on the number, size and location of breast cancer cell deposits in various regional lymph nodes, such as the armpit (axillary lymph nodes), the collar area (supraclavicular lymph nodes), and inside the chest (internal mammary lymph nodes.)[42][43] Each stage is as follows:[41]
Critical numbers of involved nodes: 1-3, 4-9, and 10 and over. Note any extranodal extension.[40]
Distant Metastases (M)
Overall stageA combination of T, N and M, as follows:[41]
|
- Further information: Evaluation of tumors
Grading
The Nottingham system[44] is recommended for breast cancer grading.[45] The Nottingham system is also called the Bloom–Richardson–Elston system (BRE)[46], or the Elston-Ellis modification[47] of the Scarff-Bloom-Richardson grading system.[48][49] It grades breast carcinomas by adding up scores for tubule formation, nuclear pleomorphism, and mitotic count, each of which is given 1 to 3 points. The scores for each of these three criteria are then added together to give an overall final score and corresponding grade as follows.
Tubule formation
By definition, tubule formation in classic invasive lobular carcinoma is scored as 3.[50]
Nuclear pleomorphism
Such as nuclei being larger, darker, or irregular/pleomorphic. Note: The cancer areas having cells with the greatest atypia should be evaluated.
- 1 point: nuclei with minimal or mild variation in size and shape
- 2 points: nuclei with moderate variation in size and shape
- 3 points: nuclei with marked variation in size and shape
Mitotic count
Mitotic figures are counted only at the periphery of the tumor, and counting should begin in the most mitotically active areas, and in at least 10 high-power fields (HPFs). If you know that the area of your high power field is about 0.2mm2, then you may score mitotic count as follows:[51]
- 1 point: ≤7 mitoses per 10 HPFs
- 2 points: 8 to 14 mitoses per 10 HPFs
- 3 points: ≥15 mitoses per 10 HPFs
If you have a significant different HPF area or you are not sure, count 10 HPFs and calculate the number of mitoses per mm2 (Further information: Evaluation#Counts per mm2 ):
- 1 point: ≤3 mitoses per mm2
- 2 points: 4 to 7 mitoses per mm2
- 3 points: ≥7 mitoses per mm2
A table of counts for various HPF sizes is available at the College of American Pathologists. [1] (Page 22)
Overall grade
The scores for each of these three criteria are added together to give a final overall score and a corresponding grade as follows:
- 3-5 Grade 1 tumor (well-differentiated). Best prognosis.
- 6-7 Grade 2 tumor (moderately differentiated). Medium prognosis.
- 8-9 Grade 3 tumor (poorly differentiated). Worst prognosis.
Microcalcifications

If invasive ductal carcinoma is seen, make at least a low power screening for microcalcifications (to correlate with imaging), but there's no need to look carefully (as tiny microcalcifications would unlikely correlate with imaging anyways).
Immunohistochemistry
Look at local protocols for what immunohistochemistry tests and other biomarker test need to be tested for each case of invasive breast cancer, or what needs to be retested for subsequent excisions at the primary site or at metastatic sites.[note 8]
Ki-67 index
Ki-67 index is mainly relevant in those with stage T1-T2, N0-N1, to determine if chemotherapy is needed (if Ki67 is >30% rather than <5%).[52]
Ki-67 index is most feasibly quantified by a hot spot method,[note 9] Hot spots are areas in which Ki-67 staining is particularly higher relative to the adjacent tumor areas.[53] Usually, the invasive edge of a tumor is a hot spot.[53] When a tumor had several hot spots, the “hottest” spot is selected.[53] Aim to count at least 500 cells in each case, but this is not always possible in cases with low tumor cell density and small tumor size.[53] Also aim to include at least three high-power (×40 objective) fields. Count a nucleus as “positive” if there is any definite brown staining in the nucleus of an invasive breast cancer cell, above the surrounding background in the cytoplasm and extracellular matrix.[54] If a comparisons must be made between core biopsies and sections from an excision, evaluation of the latter should be across the whole tumor.[52] Only nuclear staining counts. Staining intensity of a positive nucleus is not relevant.[52]
HER2
HER2 can initially be evaluated by immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH). If IHC is performed first and is borderline/equivocal, then FISH is recommended.[55] If FISH is performed first and indicates that further workup is required, then IHC may be the performed as per established algorithms.[note 10]
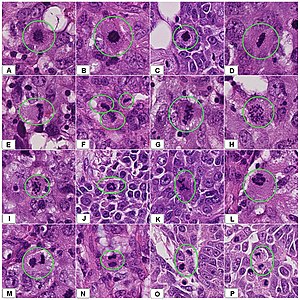
HER2 immunohistochemistry
Look at different parts of the tumor, and evaluate the area(s) with most staining. When negative of faint staining is seen, evaluate at high magnification.
| Score[56][57] | Pattern[58] | Status[56][57] |
|---|---|---|
| 0 | Either:[58]
|
HER2 negative (not present) |
| 1+ | Incomplete membrane staining that is faint or barely perceptible and within >10% of the invasive tumor cells.[58] | |
| 2+ | Weak to moderate complete membrane staining observed in >10% of tumor cells.[58] | Borderline/Equivocal |
| 3+ | Circumferential membrane staining that is complete, intense, and in >10% of tumor cells.[58] | HER2 positive |
Micrographs showing each score:[59]
HER2 FISH
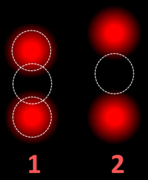
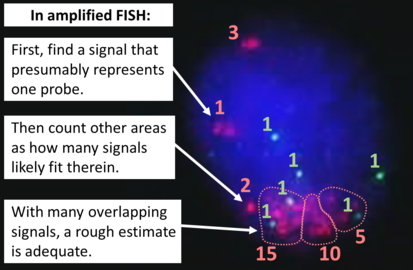
HER2 FISH usually uses chromosome enumeration probe 17 (CEP17) to count the amount of chromosomes. Hence, the HER2/CEP17 ratio reflects any amplification of HER2 as compared to the number of chromosomes.
To prepare a slide for HER2 testing, you may need to choose a paraffin-embedded and mark the resulting slide so that you or whoever interprets it knows where to look for the target tumor cells. When there are multiple blocks of the same case, choose the the one with most tumor. (If a block has undergone sectioning for immunohistochemistry (such as ER, PR and/or Ki67) make sure that you have a new H&E slide at a level next to the one to be used for FISH, so that they will correlate better.) In cases of both invasive and in situ carcinoma in the same specimen, mark all invasive carcinoma (also for crushed tissue or with other artifacts) but not the in situ carcinoma. Also mark a small area of normal tissue as an internal control. If possible, it should be a bit away from the tumor, even if only consisting of fatty tissue.
To interpret a HER2 FISH study, first perform a quality control check of the slide as per manufacturer and/or local protocol (generally including checking for proper signals from a control specimen). In cases of both invasive and in situ carcinoma in the same specimen, only score the invasive cells. The signals of 20 cells are usually counted. Also focus up and down on each nucleus to find all signals therein.
If a cytotechnologist has already performed a count, you do not have to recount, but make sure the count is reasonable regarding what you see. In any case, also look around for any obvious tumor heterogeneity in HER2 signals.
If the HER2/CEP17 ratio is borderline (1.8-2.2), count an additional 20 nuclei and recalculate a ratio for the total of 40 nuclei.
| HER2/CEP17 ratio | |||
|---|---|---|---|
| ≥2.0 | <2.0 | ||
| Average HER2 copy number per cell | ≥4.0 | HER2 positive | Additional work-up required[note 10] |
| <4.0 | Additional work-up required[note 10] | HER2 negative | |
If the initial HER2 result is negative for a needle biopsy of a primary breast cancer, a new HER2 test may be performed on the subsequent breast excision.[note 10]
Estrogen and progesterone receptors
Generally perform immunohistochemistry for estrogen and progesterone receptors and calculate the percentage of positive tumor cells.
Lobular carcinoma in situ
Fixation
Generally 10% neutral buffered formalin.
Microscopic evaluation
Lobular carcinoma in situ (LCIS) typically display monomorphic, loosely cohesive, slightly enlarged and evenly spaced cells that fill acini.[12] Cells have indistinct cell borders, pale cytoplasm, and uniform small nuclei with evenly distributed chromatin and inconspicuous nucleoli.[12]
Differential diagnosis
The main differential diagnosis is ductal carcinoma in situ (DCIS).
In DCIS, the cells are cohesive and have high grade atypia.[61]
LCIS typically fills smaller lobules rather than ducts, but DCIS can display lobular cancerization as shown at bottom of image.[image 1]
When unsure, perform immunohistochemistry for E-cadherin and p120:
In contrast, both E-cadherin (left image below) and p120 (right) have a membranous staining pattern in ductal carcinoma in situ (DCIS).
Microcalcifications

If invasive ductal carcinoma is seen, make at least a low power screening for microcalcifications (to correlate with imaging), but there's no need to look carefully (as tiny microcalcifications would unlikely correlate with imaging anyways).
Biomarkers
Testing for hormone biomarkers is not needed for LCIS (in contrast to ductal carcinoma in situ where ER/PR is generally indicated).
Microscopic report
It should contain:[62]
- Type of resection or biopsy, and location
- Results of any supplementary studies performed
- Extent
However, grading and staging is not applicable. (Margins of excision are not relevant)
Notes
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
- ↑ 2.0 2.1 An increased cold ischemia time (time from procurement to time in formalin) negatively effects the utility of immunohistochemistry.
- Khoury, Thaer (2018). "Delay to Formalin Fixation (Cold Ischemia Time) Effect on Breast Cancer Molecules ". American Journal of Clinical Pathology 149 (4): 275–292. doi:. ISSN 0002-9173. - ↑ A palpated greatest dimension before cutting is still superior to trying to align cut pieces together or mathematically adding the thicknesses of involved slices.
- ↑ Size is a major factor in whether to fully excise radial scars, at an approximate cutoff at around 1 cm.
- ↑ The proliferation of two histological components is called "biplasia", from Latin bis (“twice”) and -plasia (“formation”), or "biphasic proliferation"
- ↑ The proliferation of two histological components is called "biplasia", from Latin bis (“twice”) and -plasia (“formation”), or "biphasic proliferation" (although the latter may refer to proliferation that has two chronological phases).
- ↑ For myoepithelial markers, a combination of p63 and calponin is generally recommended for breast lesions. D2-40 is useful for highlighting lymphatics for invasion.
- ↑ If the previous biopsy was negative for ER and PR receptors, and the patient has undergone neoadjuvant chemotherapy before excision, then generally retest ER/PR on the excision. Retesting ER/PR on any excision with previously negative ER/PR on biopsy on a patient having received neoadjuvant therapy has no scientific support nor opposition.
- William M Sikov, MD, FACP, FNCBCJudy C Boughey, MD, FACSZahraa Al-Hilli, MD, FACS, FRCSI. General principles of neoadjuvant management of breast cancer. UpToDate. In breast cancer metastases, generally retest estrogen and progesterone receptors, and HER2 in the following circumstances:- If the status of the primary tumor is unknown or negative for ER/PR and/or HER2
- If the primary tumor is heterogeneous for ER/PR expression
- If the metastatic progression is unusual for the tumor characteristics
- If the relapse is unexpectedly early or late
- If unusual metastasis location
- If the initial test was performed more than 10 years ago
- If the testing turnaround time are relatively short (to reduce potential delays in patient management by retesting)
- ↑ Besides from a hot spot method of Ki67 counting, there is also a IKWG global average method which is more comprehensive. However, the inter-observer difference between the hot spot method and the 'IKWG global average is not statistically significant, and has not shown any significant difference in clinical outcome (theoretically, the area of highest Ki-67 proliferative index is probably most likely to correlate with malignant transformation and risk of metastasis, making the hot spot both more straightforward and clinically relevant than a global average).
- Reference and instructions for the IKWG global average method: Dowsett, M.; Nielsen, T. O.; A'Hern, R.; Bartlett, J.; Coombes, R. C.; Cuzick, J.; Ellis, M.; Henry, N. L.; et al. (2011). "Assessment of Ki67 in Breast Cancer: Recommendations from the International Ki67 in Breast Cancer Working Group ". JNCI Journal of the National Cancer Institute 103 (22): 1656–1664. doi:. ISSN 0027-8874. - ↑ 10.0 10.1 10.2 10.3 10.4 10.5 If additional work-up is required by FISH study, see source article for detailed algorithms:
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS (2018). "Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. ". J Clin Oncol 36 (20): 2105-2122. doi:. PMID 29846122. Archived from the original. .
Main page
References
- ↑ . Recommendations for HER2 Testing in Breast Cancer: ASCO – CAP Clinical Practice Guideline Update. College of American Pathologists (2013-10-17).
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 Monika Roychowdhury. Grossing (histologic sampling) of breast lesions. Pathologyoutlines.com. Topic Completed: 1 August 2012. Revised: 19 September 2019
- ↑ Chandler IP, Oommen R, Lawson CW (2003). "Invasive lobular carcinoma and cytokeratin immunohistochemistry: an audit. ". J Clin Pathol 56 (3): 240. doi:. PMID 12610108. PMC: 1769908. Archived from the original. .
- ↑ Mitchell, Richard Sheppard; Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson (2007). Robbins Basic Pathology (8th ed.). Philadelphia: Saunders. p. 739. ISBN 978-1-4160-2973-1.
- ↑ Jaya Ruth Asirvatham, M.B.B.S., Julie M. Jorns, M.D.. Breast - Fibrocystic changes - Sclerosing adenosis. Pathology Outlines. Topic Completed: 1 January 2015. Minor changes: 31 December 2020
- ↑ 6.0 6.1 6.2 Sofia Lérias, M.D., Melinda Lerwill, M.D.. Usual ductal hyperplasia. Pathology Outlines. Last author update: 11 February 2021. Last staff update: 25 April 2022
- ↑ 7.0 7.1 7.2 David J. Myers; Andrew L. Walls.. Atypical Breast Hyperplasia. StatPearls, National Center for Biotechnology Information. Last Update: February 15, 2019.
- ↑ Schnitt, Stuart J (2003). "The diagnosis and management of pre-invasive breast disease: Flat epithelial atypia – classification, pathologic features and clinical significance ". Breast Cancer Research 5 (5). doi:. ISSN 1465-542X.
- ↑ Logullo, Angela Flavia; Nimir, Cristiane (2019). "Columnar cell lesions of the breast: a practical review for the pathologist ". Surgical and Experimental Pathology 2 (1). doi:. ISSN 2520-8454.
- ↑ Peter Abdelmessieh. Breast Cancer Histology. Medscape. Retrieved on 2019-10-04. Updated: May 24, 2018
- ↑ 11.0 11.1 Siziopikou, Kalliopi P. (2013). "Ductal Carcinoma In Situ of the Breast: Current Concepts and Future Directions ". Archives of Pathology & Laboratory Medicine 137 (4): 462–466. doi:. ISSN 0003-9985.
- ↑ 12.0 12.1 12.2 12.3 Sucheta Srivastava. Breast - Noninvasive lobular neoplasia - LCIS classic. Topic Completed: 1 September 2017. Minor changes: 21 June 2020
- ↑ Nassar, Lara; Baassiri, Amro; Salah, Fatima; Barakat, Andrew; Najem, Elie; Boulos, Fouad; Berjawi, Ghina (2019). "Stromal Fibrosis of the Breast: A Spectrum of Benign to Malignant Imaging Appearances ". Radiology Research and Practice 2019: 1–6. doi:. ISSN 2090-1941.
- ↑ Radi MJ (1989). "Calcium oxalate crystals in breast biopsies. An overlooked form of microcalcification associated with benign breast disease. ". Arch Pathol Lab Med 113 (12): 1367-9. PMID 2589947. Archived from the original. .
- ↑ 15.0 15.1 Jaya Ruth Asirvatham, M.B.B.S., Julie M. Jorns, M.D.. Breast - Fibrocystic changes - Sclerosing adenosis. Pathology Outlines. Topic Completed: 1 January 2015. Minor changes: 31 December 2020
- ↑ Tavassoli, F.A., ed (2003). World Health Organization Classification of Tumours: Pathology & Genetics: Tumours of the breast and female genital organs . Lyon: IARC Press. ISBN 978-92-832-2412-9.
- ↑ Jaya Ruth Asirvatham, M.B.B.S., Carlos C. Diez Freire, M.D., Cansu Karakas, M.D., Belinda Lategan, M.D., Nat Pernick, M.D., Emily S. Reisenbichler, M.D., Monika Roychowdhury, M.D., Mary Ann Gimenez Sanders, M.D, Ph.D., Gary Tozbikian, M.D., Hind Warzecha, M.D. Senior Authors: Julie M. Jorns, M.D., Shahla Masood. Breast nonmalignant, Fibroepithelial neoplasms, Fibroadenoma. Retrieved on 2019-11-04. Revised: 31 October 2019
- ↑ Rosen, PP. (2009). Rosen's Breast Pathology (3rd ed.). ISBN 978-0-7817-7137-5.
- ↑ . Fibroadenoma of the breast.
- ↑ 20.0 20.1 Gary Tozbikian, M.D.. Breast - Fibroepithelial tumors - Fibroadenoma. Last author update: 19 July 2021. Last staff update: 22 July 2021
- ↑ Authors: Joshua J.X. Li, M.B.Ch.B., Gary M. Tse, M.B.B.S.. Breast - Fibroepithelial tumors - Phyllodes tumor. Pathology Outlines. Last author update: 2 December 2020. Last staff update: 11 January 2023
- ↑ Richard L Kempson MD, Robert V Rouse MD. Fibroadenoma of the Breast. Stanford University School of Medicine. May 27, 2006
- ↑ Cowan ML, Argani P, Cimino-Mathews A (2016). "Benign and low-grade fibroepithelial neoplasms of the breast have low recurrence rate after positive surgical margins. ". Mod Pathol 29 (3): 259-65. doi:. PMID 26743469. Archived from the original. .
- ↑ Rageth, Christoph J.; Rubenov, Ravit; Bronz, Cristian; Dietrich, Daniel; Tausch, Christoph; Rodewald, Ann-Katrin; Varga, Zsuzsanna (2018). "Atypical ductal hyperplasia and the risk of underestimation: tissue sampling method, multifocality, and associated calcification significantly influence the diagnostic upgrade rate based on subsequent surgical specimens ". Breast Cancer 26 (4): 452–458. doi:. ISSN 1340-6868. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/)
- ↑ 25.0 25.1 Tozbikian, Gary; Brogi, Edi; Vallejo, Christina E.; Giri, Dilip; Murray, Melissa; Catalano, Jeffrey; Olcese, Cristina; Van Zee, Kimberly J.; et al. (2016). "Atypical Ductal Hyperplasia Bordering on Ductal Carcinoma In Situ ". International Journal of Surgical Pathology 25 (2): 100–107. doi:. ISSN 1066-8969.
- ↑ Sucheta Srivastava, M.D.. Breast - Noninvasive lobular neoplasia - LCIS classic (Differential diagnosis section). Topic Completed: 1 September 2017. Minor changes: 17 May 2021
- ↑ . Protocol for the Examination of Resection Specimens from Patients with Ductal Carcinoma In Situ (DCIS) of the Breast, Version: 4.4.0.0. Protocol Posting Date: June 2021. College of American Pathologists.
- ↑ Image annotation by Mikael Häggström, MD, using source image from:
Moatasim A, Mamoon N (2022). "Primary Breast Mucinous Cystadenocarcinoma and Review of Literature. ". Cureus 14 (3): e23098. doi:. PMID 35464581. PMC: 8997314. Archived from the original. .
- "This is an open access article distributed under the terms of the Creative Commons Attribution License CC BY 4.0."
Source for microinvasion: . Protocol for the Examination of Resection Specimens from Patients with Ductal Carcinoma In Situ (DCIS) of the Breast, Version: 4.4.0.0. Protocol Posting Date: June 2021. College of American Pathologists. - ↑ Gary Tozbikian, M.D.. Breast - Ductal carcinoma in situ - DCIS. pathologyOutlines. Topic Completed: 20 May 2020. Minor changes: 6 May 2021
- ↑ . Ductal Carcinoma in Situ of the Breast. Stanford Medical School (2020-08-27).
- ↑ Image by Mikael Häggström, MD.
- Reference for most common definition of comedo necrosis by size:
- Harrison BT, Hwang ES, Partridge AH, Thompson AM, Schnitt SJ (2019). "Variability in diagnostic threshold for comedo necrosis among breast pathologists: implications for patient eligibility for active surveillance trials of ductal carcinoma in situ. ". Mod Pathol 32 (9): 1257-1262. doi:. PMID 30980039. Archived from the original. . - ↑ Image by Mikael Häggström, MD. References for features:
- . Ductal Carcinoma in Situ of the Breast. Stanford Medical School (2020-08-27).
- Hayward MK, Louise Jones J, Hall A, King L, Ironside AJ, Nelson AC (2020). "Derivation of a nuclear heterogeneity image index to grade DCIS. ". Comput Struct Biotechnol J 18: 4063-4070. doi:. PMID 33363702. PMC: 7744935. Archived from the original. . - ↑ Hayward MK, Louise Jones J, Hall A, King L, Ironside AJ, Nelson AC (2020). "Derivation of a nuclear heterogeneity image index to grade DCIS. ". Comput Struct Biotechnol J 18: 4063-4070. doi:. PMID 33363702. PMC: 7744935. Archived from the original. .
- ↑ 34.0 34.1 Allred, D. C. (2010). "Ductal Carcinoma In Situ: Terminology, Classification, and Natural History ". JNCI Monographs 2010 (41): 134–138. doi:. ISSN 1052-6773.
- ↑ Laura C Collins, MD, Christine Laronga, MD, FACS, Julia S Wong, MD. Ductal carcinoma in situ: Treatment and prognosis. UpToDate. Literature review current through: Sep 2022. | This topic last updated: Aug 19, 2022.
- ↑ Top and bottom left images by Mikael Häggström, MD. Bottom right image from:
Kulka J, Madaras L, Floris G, Lax SF (2022). "Papillary lesions of the breast. ". Virchows Arch 480 (1): 65-84. doi:. PMID 34734332. PMC: 8983543. Archived from the original. .
- "This article is licensed under a Creative Commons Attribution 4.0 International License" - ↑ "Association of Infiltrating Lobular Carcinoma With Positive Surgical Margins After Breast-Conservation Therapy ". Ann. Surg. 231 (6): 877–82. June 2000. doi:. PMID 10816631.
- ↑ "Synchronous bilateral invasive lobular breast cancer presenting as carcinomatosis in a male ". Am. J. Surg. Pathol. 33 (3): 470–4. March 2009. doi:. PMID 19092630.
- ↑ Fletcher's diagnostic histopathology of tumors. 3rd Ed. p. 931-932.
- ↑ 40.0 40.1 40.2 40.3 40.4 . Infiltrating Ductal Carcinoma of the Breast (Carcinoma of No Special Type). Stanford Medical School. Retrieved on 2019-10-02.
- ↑ 41.0 41.1 41.2 Originally copied from Fadi M. Alkabban; Troy Ferguson. Cancer, Breast. National Center for Biotechnology Information. Last Update: June 4, 2019. Creative Commons Attribution 4.0 International License
- ↑ "Internal mammary lymphadenopathy in breast carcinoma: CT appraisal of anatomic distribution ". Radiology 167 (1): 89–91. April 1988. doi:. PMID 3347753.
- ↑ "Internal mammary lymphadenopathy: imaging of a vital lymphatic pathway in breast cancer ". Radiographics 10 (5): 857–70. September 1990. doi:. PMID 2217975.
- ↑ Elston, CW; Ellis, IO (1991). "Pathologic prognostic factors in breast cancer. I. The value of histological grades in breast cancer. Experience from a large study with long-term follow-up ". Histopathology 19 (5): 403–10. doi:. PMID 1757079. "Republished ". Histopathology 41: 154–161. 2002. doi:.
- ↑ Oudai Hassan. What is the Nottingham combined histologic grade (modified Scarff-Bloom-Richardson grade) system for breast tumors?. Medscape. Updated: Mar 20, 2019
- ↑ Al-Kuraya, Khawla; Schraml, Peter (2004). "Prognostic relevance of gene amplifications and coamplifications in breast cancer ". Cancer Research 64 (23): 8534–8540.
- ↑ Elston CW, Ellis IO. Pathologic prognostic factors in breast cancer. I. The value of histological grades in breast cancer. Experience from a large study with long-term follow-up. Histopathology 1991, 19:403-410.
- ↑ Bloom, H.J.; Richardson, W.W. (1957). "Histological grading and prognosis in breast cancer; A study of 1409 cases of which 359 have been followed for 15 years ". British Journal of Cancer 11 (3): 359–77. doi:. PMID 13499785.
- ↑ Genestie, C.; Zafrani, B.; Asselain, B.; Fourquet, A.; Rozan, S.; Validire, P.; Vincent-Salomon, A.; Sastre-Garau, X. (1998). "Comparison of the prognostic value of Scarff-Bloom-Richardson and Nottingham histological grades in a series of 825 cases of breast cancer: Major importance of the mitotic count as a component of both grading systems ". Anticancer Research 18 (1B): 571–6. PMID 9568179.
- ↑ . Infiltrating Lobular Carcinoma of the Breast. Stanford Medicine. Retrieved on 2021-01-06.
- ↑ Error on call to Template:cite web: Parameters url and title must be specified. . College of American Pathologists. Protocol Posting Date: September 2022
- ↑ 52.0 52.1 52.2 Dowsett, M.; Nielsen, T. O.; A'Hern, R.; Bartlett, J.; Coombes, R. C.; Cuzick, J.; Ellis, M.; Henry, N. L.; et al. (2011). "Assessment of Ki67 in Breast Cancer: Recommendations from the International Ki67 in Breast Cancer Working Group ". JNCI Journal of the National Cancer Institute 103 (22): 1656–1664. doi:. ISSN 0027-8874.
- ↑ 53.0 53.1 53.2 53.3 Coleman, William B.; Jang, Min Hye; Kim, Hyun Jung; Chung, Yul Ri; Lee, Yangkyu; Park, So Yeon (2017). "A comparison of Ki-67 counting methods in luminal Breast Cancer: The Average Method vs. the Hot Spot Method ". PLOS ONE 12 (2): e0172031. doi:. ISSN 1932-6203.
- ↑ . Ki67-QC international working group: whole section scoring protocol (global method). International Ki67 in Breast Cancer Working Group (2018-11-29).
- ↑ . Breast Cancer HER2 Status. American Cancer Society. Last Revised: August 25, 2022
- ↑ 56.0 56.1 . IHC Tests (ImmunoHistoChemistry). Breastcancer.org. Last modified on October 23, 2015
- ↑ 57.0 57.1 "Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications ". Molecular Biology International 2014: 852748. 2014. doi:. PMID 25276427.
- ↑ 58.0 58.1 58.2 58.3 58.4 2018 ASCO/CAP guidelines:
- . Figure 1. Algorithm for evaluation of human epidermal growth factor receptor 2 (HER2) protein expression by immunohistochemistry (IHC) assay of the invasive component of a breast cancer specimen.. College of American Pathologists: Homepage. Retrieved on 2022-09-12.
- Ahn S, Woo JW, Lee K, Park SY (2020). "HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation. ". J Pathol Transl Med 54 (1): 34-44. doi:. PMID 31693827. PMC: 6986968. Archived from the original. . - ↑ Nitta H, Kelly BD, Padilla M, Wick N, Brunhoeber P, Bai I (2012). "A gene-protein assay for human epidermal growth factor receptor 2 (HER2): brightfield tricolor visualization of HER2 protein, the HER2 gene, and chromosome 17 centromere (CEN17) in formalin-fixed, paraffin-embedded breast cancer tissue sections.
". Diagn Pathol 7: 60. doi:. PMID 22647525. PMC: 3487810. Archived from the original. .
- "This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0)" - ↑ Diagram and table by Mikael Häggström, MD. Adapted from: Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS (2018). "Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. ". J Clin Oncol 36 (20): 2105-2122. doi:. PMID 29846122. Archived from the original. .
- ↑ Sucheta Srivastava, M.D.. Breast - Noninvasive lobular neoplasia - LCIS classic (Differential diagnosis section). Topic Completed: 1 September 2017. Minor changes: 17 May 2021
- ↑ . Lobular Carcinoma in Situ of the Breast. Surgical Pathology Criteria. Retrieved on 2021-12-14.
Image sources
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Image(s) by: Mikael Häggström, M.D. Public Domain
- Author info
- Reusing images