Starting pathology (entire handbook)

Author: Mikael Häggström, M.D.
- Conflicts of interest: None. (No financial gain was or is made from this handbook, apart from the academic prestige of being an author.)
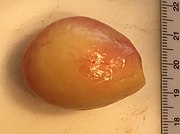
Front page images by Mikael Häggström, M.D., except picture of sectioned heart, which is from: Michaud, Katarzyna; Basso, Cristina; d’Amati, Giulia; Giordano, Carla; Kholová, Ivana; Preston, Stephen D.; Rizzo, Stefania; Sabatasso, Sara; et al. (2019). "Diagnosis of myocardial infarction at autopsy: AECVP reappraisal in the light of the current clinical classification ". Virchows Archiv. doi:. ISSN 0945-6317. This picture is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/)
This is a handbook directed at new pathology trainees (mainly residents or trainees with medical doctor degrees or similar). The main goal of this handbook is to provide you with the most pertinent knowledge in order to most efficiently improve the lives of people through better diagnoses and thereby better treatment.
Table of contents
- Memorization-worthy:[note 1] Have an idea of the contents herein, so that you know what you can look for when you have such a case.
Test question
You receive five pathology cases, and feel that you want to look up what to do when you prepare them and subsequently evaluate them under the microscope. Which one does not have a dedicated article in Starting pathology?
|
Contents
- 1 Table of contents
- 2 Copies, prints and reuse
- 3 Contact
- 4 Learning pathology
- 5 What a pathologist needs to memorize
- 6 Using resources
- 7 Test question
- 8 Using this resource
- 9 Using question banks
- 10 Learning clinical pathology
- 11 General advice
- 12 Comprehensiveness
- 13 Emergent pathology
- 14 Fixation
- 15 Gross processing
- 16 Evaluation
- 17 Cytology introduction
- 18 Tumors, introduction
- 19 Evaluation of suspected malignancies
- 20 Metastasis
- 21 Immunohistochemistry
- 22 Consultation
- 23 Reporting
- 24 Gastrointestinal pathology
- 25 Appendix
- 26 Appendicitis
- 27 Gallbladder
- 28 Cholecystitis
- 29 Gallbladder polyp
- 30 Endoscopic gastrointestinal biopsies
- 31 Esophagus
- 32 Eosinophilic esophagitis
- 33 Gastroesophageal junction
- 34 Barrett's esophagus
- 35 Esophageal adenocarcinoma
- 36 Stomach
- 37 Gastric polyp
- 38 Fundic gland polyp
- 39 Gastritis
- 40 Stomach biopsy for Helicobacter pylori
- 41 Stomach tumor
- 42 Gastric sleeve
- 43 Duodenum
- 44 Small intestine in celiac disease
- 45 Colorectal polyp
- 46 Hyperplastic polyp
- 47 Tubular and ⁄or villous adenoma
- 48 Sessile serrated adenoma
- 49 Traditional serrated adenoma
- 50 Breast pathology
- 51 Breast biopsy or excision
- 52 Fibrocystic breast changes
- 53 Fibroepithelial tumor
- 54 Atypical ductal hyperplasia
- 55 Invasive ductal carcinoma
- 56 Invasive lobular carcinoma
- 57 Lobular carcinoma in situ
- 58 Autopsy
- 58.1 Checklist for non-forensic autopsy
- 58.1.1 Preparations
- 58.1.2 External inspection
- 58.1.3 In situ inspection
- 58.1.4 Evisceration
- 58.1.5 Organ packages
- 58.1.6 Heart
- 58.1.7 Other thorax
- 58.1.8 Retroperitoneal
- 58.1.9 Peritoneal
- 58.1.10 Brain
- 58.1.11 Demonstration
- 58.1.12 Weighing
- 58.1.13 Tissue sampling
- 58.1.14 Microbiology
- 58.1.15 Toxicology
- 58.2 Reporting of non-forensic autopsy
- 58.3 External applications
- 58.1 Checklist for non-forensic autopsy
- 59 Heart autopsy
- 60 Autopsy of myocardial infarction
- 61 Ruptured intercalated discs
- 62 Lung autopsy
- 63 Kidney autopsy
- 64 Brain autopsy
- 65 Reproductive system
- 66 Cervical biopsy
- 67 Cervical cone
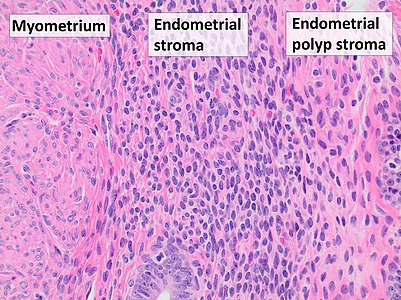
- 68 Endometrial polyp
- 69 Endometrial curettings
- 70 Endometrial thickening
- 71 Hysterectomy
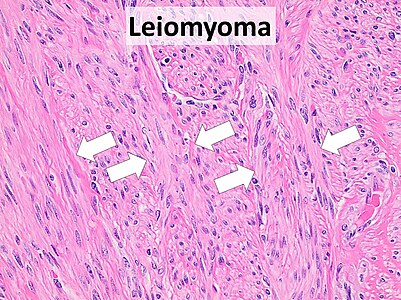
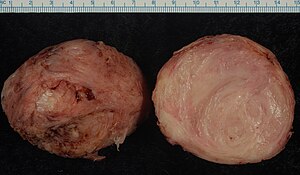
- 72 Smooth muscle tumor
- 73 Intrauterine device
- 74 Products of conception
- 75 Fallopian tube
- 76 Ovary
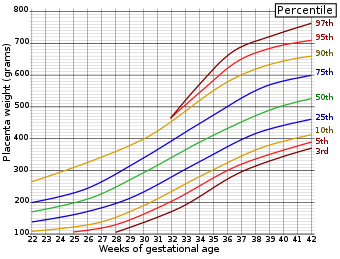
- 77 Placenta
- 78 Vulva
- 79 Cervical cytology
- 80 Male reproductive system
- 81 Phimosis
- 82 Vas deferens
- 83 Skin
- 84 Notes
- 85 References
Copies, prints and reuse
To get a printable version of this handbook (which can also be downloaded for offline use), go to patholines.org, click Starting pathology (handbook), and click Printable version in the left hand menu.[note 2] A normal color printer and normal paper is sufficient, and you can keep the pages together in a binder. If you want even better quality, use color printer paper, or google a "copy shop" for a color print delivery or in-store pickup (possibly as a photo book). If you are not intending to practice in clinical pathology (or laboratory medicine) then you may skip printing the "Clinical pathology" part.
This handbook is released under the Creative Commons Attribution 4.0 International (CC-BY 4.0) license, meaning that you may reuse any of its content (such as in scholarly articles or other publications of yours), but you should mention its author (Mikael Häggström, M.D.). Also, many images are Public Domain, that is, without any restrictions. The status of each image, and its creator(s), is seen on its description page (found by clicking the image at Patholines.org). This handbook is published in the United States, and the United States copyright law holds that "In no case does copyright protection for an original work of authorship extend to any idea, procedure, process, system, method of operation, concept, principle, or discovery, regardless of the form in which it is described, explained, illustrated, or embodied in such work.".[1][2] Hence, you can use any texts, tables and graphs herein as Public Domain, and defend such usage by stating that it describes anatomical, physiological and pathological processes and systems. Hence, in practice, the attribution as per above when using texts, tables and graphs from this handbook is a polite request rather than a legal requirement.[3]Further information: Patholines:Copyright
Contact
To contact the page creator/author, write a message to mikael![]() haeggstroem
haeggstroem![]() nuvancehealth
nuvancehealth![]() org.
org.
Please give feedback on any errors that you encounter in this resource, as well as pertinent omissions that you feel should be there. Preferably, contribute yourself: See the Contribute page at Patholines.org. This is a not-for-profit textbook, so there are no monetary rewards for contributing. Yet, a major co-author or reviewer may be eligible for inclusion on the front page. Contributors will otherwise be mentioned on a second page (with details on what parts were written or reviewed), unless requesting to remain anonymous. Contributors may also mention their efforts on their professional resumes.
Learning pathology
The goal of this resource is to make a new pathology trainee able to properly handle at least 70% of cases that are expected at an average general pathology department, including the exclusion of the most pertinent differential diagnoses thereof. Its aim is to present you at least one way of doing things that is at least adequate for a particular situation, so that although there may be various routines at various locations, adherence to these methods should not result in a worse reprimand from a senior than "although that was adequate, that is not how we do it here". Still ask for help whenever needed, such as first time you are doing something, or whenever you are not sure about what to do, especially when doing something potentially irreversible.
What a pathologist needs to memorize
The best method for memorization is generally through repeated exposure in everyday practice, and the scope thereof will depend on your eventual location and subspecialty, and you will eventually forget everything else, more or less. Yet, the following things are most important for a pathologist trainee to focus on memorizing:
- Emergency pathology, mostly relating to intraoperative or frozen section consultations. This includes information that usually cannot be timely looked up on the Internet when needed.
- Main pitfalls: The most common and dangerous situations where a pathologist may not recognize the need to look something up further or ask a senior colleague, or where a significant risk of diagnosis error remains after doing so.
- Interpretation of non-written results, including patterns and signs which can be seen grossly or under the microscope. It confers the ability to translate visuals and other more or less non-verbalizable information into words that can be looked up if needed, and conceive the most likely diagnoses based on both describable and more abstract appearances.
- Proficiency in diagnosing equivocal or borderline cases where readily available sources and evidence usually deal with discrete and specific disease entities and subcategories thereof. Unusual or equivocal presentations of very common diseases and conditions are still generally more common than rare diseases, and constitutes a major workload in everyday pathology practice. However, most textbooks still give disproportionately large room for rare diseases compared to such presentations. Nevertheless, strive to master the common conditions (including the most common pitfalls) first, and skip the uncommon in the meantime. After all, you will get by with effectively handling the regular cases wherever you work, so that when unfamiliar cases appear, you will have time to study or ask others for how to handle them, as long as you know your pitfalls, and have an idea about your unknowns:
- Having an idea of one’s unknowns; being aware of a lack of knowledge in unfamiliar fields. For example, a pathologist generally does not need in depth knowledge about diseases that are generally sent out to specialized centers (such as pediatric musculoskeletal oncology), but just enough to be able to suspect such diseases. In the same manner, a pathologist needs to have an idea of the optimal limits of practice, between what should optimally be handled personally by the pathologist and what is better handled by others, including senior colleagues, technicians, pathology assistants, junior colleagues and artificial intelligence.
- Where to find information for various situations. It includes which person or which search engine is most useful for various clinical situations. See the Using resources section below for further information. Similarly, a pathologist often needs to be efficient at finding what is relevant among a large amount of information, and be able to integrate it into a decision or a relatively short report. Experience in looking up information will eventually give you an idea of where to locate specifics that you haven't even seen before. For questions where a look-up does not readily give the answer, a new trainee usually gets satisfactory replies even from senior trainees, but the more experienced you get, the more you need to establish contacts with particular people, such as certain subspecialties, who are most likely to be able to answer your questions when needed.
- How to distinguish memorization-worthy versus look-up versus practically useless information. With the ease of access to pathology information on the Internet through smartphones and computers, those studying for the everyday practice as a pathologist should not waste time memorizing what can essentially always be conveniently and timely looked up when needed. This includes most of the content of books and web pages that are sorted by names of diseases and conditions, because if the name of a disease is already known, then it can relatively quickly be looked up when needed in such sources. When finding relevant look-up information on the Internet or local repositories, it is generally enough to remember enough of its related words in order to find it again. After all, other doctors and even laypersons can look up diseases and conditions themselves, without the need for a pathologist consultation, so the expertise of memorizing such readily available information is expendable. Similarly, electronic systems are better suited than the human brain for the storage and rapid retrieval of databases of for example immunohistochemistry and genetics results of various diseases. The topics listed in this section are already immense enough to cover a lifetime of learning. Learning pathology therefore optimally involves the memorization of where and how to find look-up information, rather than directly memorizing such information. Therefore, if you need to choose a textbook to study, then choose one that you will always be able to quickly reach through your smartphone whenever you need its look-up information. Accordingly, the final aim of this resource is to help pathology trainees to give accurate and clinically useful reports to clinicians, in synergy with the resources that are available in everyday pathology practice, and to establish routines to continue such practices. The intended end result is to help clinicians to improve the lives as much as possible to as many people as possible, even if it means forgoing the prestige of becoming a walking encyclopedia. After all, life is not a Trivial Pursuit game; Don't fill your brain with random facts, even pathology-related ones. Rather, use high-yield learning sources, for even if they may take longer time to find, you will be better prepared for real life than when you study low-yield knowledge. For instance, the optimal studying is generally not of diseases individually, but rather of patterns of signs and other findings and the likelihoods of each differential diagnosis thereof. Arguably the best method of acquiring such knowledge is to be involved in many real cases at a pathology department, making your own diagnostic thinking of them, and correcting your thinking based on how you differed from a senior's decision on the case. Real life experience also gives you a better idea of what is memorization-worthy versus look-up information in various resources. After real life experience, the next best resource is arguably to solve virtual cases such as found in pathology qbanks.
As a general rule, the following factors makes a piece of information a look-up one:
- The probability of realizing that you don't know it in situations where it makes a difference. For example, when being presented with a numerical test result and you do not know the normal reference range for it, you at least realize that there are reference ranges out there that you can look up to help you. Thus, reference ranges are generally look-up information. It is generally the opposite for pitfalls. For example, if a person thinks a breast nodule looks like benign fibromatosis but does not know (or does not consider) that fibromatosis-like metaplastic carcinoma (FLMBC) looks similar, the probability is relatively low that the person will look that up.[note 3]
- The ease and speed of looking it up in reliable sources. Decisions with higher patient impact require more reliable sources. For example, the first forum answer from an anonymous user can be sufficient for an inconsequential decision, whereas information that may make a diagnosis benign versus malignant generally needs to be found on credible and peer reviewed sites.
- How to efficiently study for exams that contain questions that require direct memorization of look-up (or even practically useless) information, as long as such exams are in the way of practicing pathology. The best approach is arguably to spend enough time and effort on memorizing what is memorization-worthy, to the point where you will pass an exam even if you will fail multiple questions that require memorization of look-up or practically useless information. However, when starting pathology training, do not focus on studying for any final exams that are years away, because the content on such exams is systematically very different from what you need to know initially. For instance, you initially need to know how to handle the common types of cases, but exam makers on the other hand, in their effort to distinguish those who have studied more extensively than others, will systematically choose presentations that are not commonly encountered. Further information: Secrets Further exam advice is found in the Secrets chapter at patholines.org.
| Type of information | Examples (usually) | Learning approach |
|---|---|---|
| Memorization-worthy |
|
Directly memorizing it. |
| Look-up |
|
Knowing where to find it when needed, and how to use it. |
| Low-yield |
|
Instead studying patterns of findings and signs, and how they support various differential diagnoses. |
| Practically useless |
|
Avoiding, or skipping to potentially useful parts. |

Whether any piece of information is useful versus useless depend mainly on:
- The likelihood of a situation where the information is useful.
- The benefit versus detriment of having versus not having the information at hand in such a situation.
The clinical management aspects that can be useful for pathologists to learn are the thresholds for pathologic findings that substantially change the clinical management, such as a cancer stage that determines whether clinicians will try to completely remove all tumors versus treat palliatively. With such knowledge, pathologists can put relatively more effort on the cases and parts thereof that make the most difference to the patient. Otherwise, clinical management knowledge is generally useless to a pathologist.
Lectures may be memorization-worthy depending on the content, but a lecture of look-up-information is practically useless unless you maintain a system to quickly retrieve the information when needed. Thus, sometimes, it is better to study something more memorization-worthy while pretending to listen to a lecturer.
Also keep in mind that pathology in our look-up era is mainly a processing work rather than a memorization one, since your most important skills are to interpret findings, and sometimes involve a vast amount of information in order to suggest the most likely diagnoses. Again, arguably the best method fur such purposes is solving real or virtual cases.
Using resources
This resource is written with the intention to teach you what to do in the most common situations you are expected to encounter during your first years of pathology training, at least until the point that you are usually fairly confident about what disease or condition you have at hand, because then you know what words to use to look it up in the vast literature out there. At that point, the fastest way to get more information is generally by Googling the disease or condition name, followed by pathology outlines (which is generally the most likely to quickly give you the information you need), since Internet will always be readily available in pathology.[note 4] The most efficient method of studying to solve a case at hand is to not read articles in their entirety, but to scroll directly to the parts that are most likely to give you the information you need (generally past the first half of Pathology Outlines articles). A major skill of a pathologist is to have a good idea of what pages and texts can be skipped by only a quick glance.
Specific search methods for some specific purposes include the following (and the author has no financial or other conflicts of interests in mentioning any of these):
- Google, and then clicking the Images tab, if you just want to see more micrographs of the disease or condition. Even when you don't have a clue what condition you are looking at, you may find something that looks similar to your case by entering words you would use for the microscopic findings that you see.
- Adding cancer.net staging in Google searches for definitions of cancer stages, for example Googling prostate cancer cancer.net staging. The first search will then generally be the one from the American Society of Clinical Oncology.
- Googling "CAP synoptics" for guidelines for the most common cancer locations and types [1]. Don't google individual protocols, bypassing the overview list of protocols, as it may cause you to click on an outdated protocol.
- Considering a subscription for ImmunoQuery[note 5] (or equivalent database if you find one) in order to generate the most pertinent immunohistochemistry stains when you have two or more differential diagnoses for a case at hand. It is not sufficient to just memorize a few usually positive or negative immunostains for each disease and condition, because what you need in reality is to figure out what immunostains to order so that you can pinpoint one diagnosis among your multiple differentials. To master that, you will need to memorize a vast amount of immunostains and at what percentages they are positive for a vast amount of diseases and conditions, or you can have an online service like ImmunoQuery do that work for you. After all, in pathology there is essentially no immunohistochemistry that is so emergent that you do not have the time to use a computer or smartphone to look it up.
- ClinVar to look up the pathogenicity of specific genetic variants/mutations.
If the above resources do not provide a sufficient answer, more general search tools are mainly:
- Googling what you are looking for. You may add the word pathology or equivalent if results are too clinical or layman-oriented. Before even reading the title of results, first look at the URL. If it is from a reputable organization then you may proceed to check if the title is pertinent to what you are looking for, but if it is from an unfamiliar site then you should only proceed if you don't find a better source among your search results. On such unfamiliar sites, you will need to look for additional proof of reliability such as authorship of the content in order to use it as part of any diagnostic decisions.
- ChatGPT is useful in answering specific questions, or generating texts with particular content or format. However, don't make significant diagnostic decisions based on its answers without first verifying it with reliable sources, as it might give a false answer despite appearing confident.
You can generally rely on books and articles for presumably well-established topics (such as anatomy, and relatively common diseases). However, for potentially controversial and experimental topics, you generally need to ask yourself if the author has a conflict of interest before using its information for important decisions.
Ask a colleague at least whenever your own memory or a resource search is not enough, and there is a significant risk that you may do something irreversible that will negatively affect a patient.
Regarding lectures and knowledge your colleagues will tell you, make a judgment for each piece of information if it fits a criterion for memorization, or if it merits being written down somewhere you can find it so that you can look it up when needed.
Wikipedia often shows up among top Google results, and is generally accurate.[5]
Whenever possible, use textbooks and other sources that you can quickly access online, because you will likely always be close to the Internet, but not always close to your collections of physical material. Keep in mind that for the vast majority of content, you only need to have a hunch of where to find it again when needed, or what search terms to use. If something may be difficult to find again, you may add it to a personal digital collection to be readily available in times you need it.
Strive for sources that, taken together, are comprehensive enough for the presentation at hand. For example, if you first look in a resource on only benign conditions that may cause your presentation, you generally need to look for another source that includes malignant conditions as well.
Generally skip any part that tries to explain a microscopic appearance using only words, since a Google image search is generally more useful.
Test question
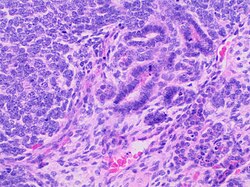
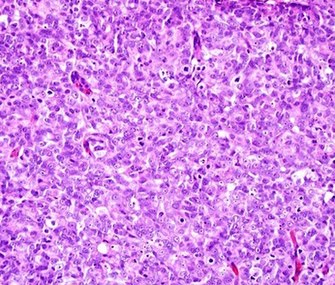
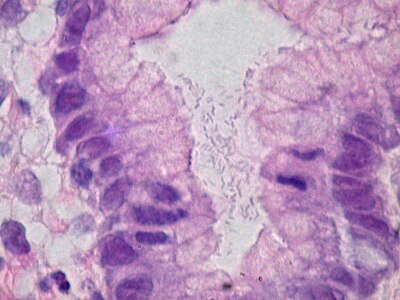
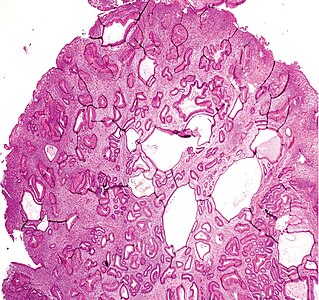
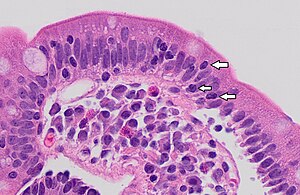
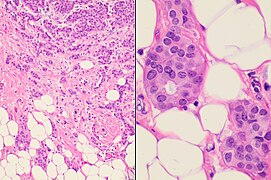
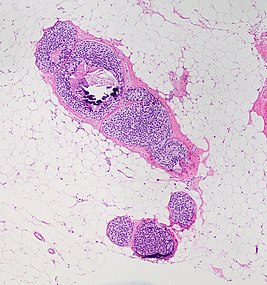
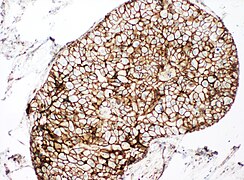
You are a new resident at a community hospital that is closely affiliated with a university hospital. The attending gives you a couple of slides from a case that is not yet signed out to preview and then come back with what you think would be the next steps. You are also told to share them with a med student who rotates at the department. One of the cases is from a kidney of a 4 year old girl. You look up the patient's history and she was admitted for orthopedic care for multiple fractures, and CT incidentally found a kidney tumor where workup was initiated as an inpatient. Microscopy shows the image at right. The med student has a smartphone and by googling "common kidney tumors in children", Wilms' tumor (also known as nephroblastoma) shows up as one of the top choices. You look up the condition on Pathology Outlines and the case seems to fit the microscopic description with its 3 typical components (second image at right).[6] On the same web page you also quickly see the following pieces of look-up information:
Which of the following would be the best next step if you were to decide yourself?
A major skill of a pathologist is having an idea of one’s unknowns. Furthermore, pediatric oncology is generally outside the limits of independent practice of any individual pathologist, generally requiring at least consultation with a colleague before diagnosis, classification and decisions about further workup (which in this case may be made at a pediatric oncology center). Thus, even if you though you knew how to proceed on your own, saying "I don't know" and asking for help is still the most correct as a new resident for a case like this.
You can skip reading these if you are already agree with the explanation above.
|
Using this resource
In this resource, it is recommended to read attentively until the end of the emergent pathology section. That part can be regarded as mainly describing concepts, which are relevant to read through and understand or at least consider. The rest will mainly provide guidelines for various situations, such as when you are presented with a biopsy of the gastrointestinal tract. Memorization-worthy information will generally be highlighted by: Memorization-worthy:[note 6], otherwise it is recommended to scroll quickly through such situation-based chapters, just to get an idea of what kind of information is available there, and then keeping this resource at a known location for whenever you are in that situation.
- Memorization-worthy:[note 7] This resource is available open-access and ad-free by googling patholines and then Starting pathology (handbook).
This resource is aimed at presenting the most important knowledge that you will need when starting pathology. For further knowledge that you will need as a pathologist and any subspecialization, the best way is to learn from reality, by solving the cases and problems that get presented to you, and thereby look specifically for the information that you will need among the immense literature and other information that is out there.
Also, this resource assumes that you use additional sources when needed (such as The WHO Classification of Tumors or others in the Using resources above). Therefore, it will not simply repeat such information, but may display external links to further information.
This resource also assumes that you will ask a colleague for advise if you see an unfamiliar presentation, and any literature lookup thereof still does not explain it. Therefore, this resource will focus on the most common presentations and the main differential diagnoses thereof, which may include rare conditions if the clinical management thereof would be significantly different. On the other hand, this resource omits rare conditions that rarely look similar to common presentations. Therefore, keep in mind that there are many such rare conditions that you do not know about, so keep asking colleagues whenever a presentation remains strange to you, and choose initial workplaces where you have experienced people to ask if needed.
Be tolerant to encountering many repetitions of the same content in related articles, since for example a cervical biopsy and a cervical cone have similar microscopic evaluation.
Using question banks
To test your knowledge on memorization-worthy information, seek questions that present you with a possible real life situation, as well as all pertinent information you can readily look up before needing to answer the question, so to ensure that it tests you on pertinent information as per the items earlier in this chapter. On the other hand, don't waste time on questions whose answer requires memorization of look-up or practically useless information (except if you want to practice looking up the answers online). There are about 5700 questions in PathPrimer, PathDojo, BoardVitals, and the ASCP Question Bank combined,[note 8] so you can be picky about which questions to take and which ones to skip.
Consider the usefulness of each question before spending significant time memorizing its answers or explanations, in order to maximize the useful knowledge you will learn overall. Generally, skip questions that test you on look-up information and practically useless information. Whenever you have a question where you can not picture in what real life situation it would be relevant to remember, then you can generally assume that it never will be. For questions that consists of a useful item together with a more useless one, only learn the the useful part and move on. If you can, flag/mark questions on memorization-worthy information that you did not know, and move on fairly quick on those as well, and later revisit those questions specifically if you have the time, as repetition is generally more efficient than lingering.
A common sign that a question will deal with look-up or practically useless information is where it asks which of the alternatives is false or correct for one particular condition, and the alternatives are relatively heterogenous (such as one dealing with diagnosis and another one dealing with prognosis). The high prevalence of such questions is mainly because they are easy and quick create, because the question creator can simply look up a condition, choose some more or less random facts, and then change small details to create alternatives. It requires no real life experience in everyday pathology practice, and learning from such a question is hence generally not useful for that purpose either.[note 9] The thought processes of real life pathology is generally the opposite of this, because you are generally presented with a specific location, gross appearance and microscopic appearance, and you need to practice on finding the most likely causative condition thereof among many differential diagnoses. Even other kinds of memorization-worthy pieces of information generally have a more limited scope among alternatives (like knowing the optimal staining duration in hematoxylin for a frozen section among alternative durations (see Emergent pathology). Thus, to practice your knowledge in memorization-worthy pathology, it is generally most efficient to skip false-versus-correct questions, in order to spend your limited time on something more worthwhile. Similarly, questions are in general rather low-yield whenever they deviate from the realistic direction.
Also, prefer succinct questions, that give you only the most relevant information in order to test the key learning point, without having to spend effort finding the most relevant information in long patient presentations, because you will get enough practice of that in everyday scrolling through patient charts.
If you have gone through all 5000 questions of the main question banks, and still have plenty of time for a second round, then you may work on lower yield questions as well. For example, you may practice on looking up answers for look-up questions, unless the presentation makes it clear that you cannot timely and conveniently do so (such as being in a rush during a frozen section).
Test question
You want to practice your memorization-worthy knowledge in real life pathology and not for exams (Further information: Secrets 'as per the Secrets chapter at patholines.org.). You find the following multiple choice question: "Which of the following statements for squamous cell carcinoma (SCC) of the skin is false?"
What is the most efficient way of dealing with this question for real-life pathology practice?
You can skip reading these if you are already agree with the explanation above.
|
Other indications for skipping a question include:
- Unrealistically withholding information, such as mentioning what kind of test identified the condition without giving you the test result (such as a case presentation that includes "Serology identified the organism").
- Unrealistic chronology, that is, not following the order of actual processes. It may include giving you the diagnosis and asking you what would be the expected clinical history.
- Showing you a molecular structure and asking you to identify the substance or its category, which is extremely unlikely to be a task in real life, and which can generally quickly be looked up (such as googling search by image and clicking the camera icon, followed by uploading the image of the structure, such as a photo or screenshot thereof).
- Unpractical statistics that is very unlikely to be used diagnostically, mainly because their calculations would be so complex that it is not worth it. For example, in reality you generally have a pattern of signs and other findings and you want to know the likelihood of each differential diagnosis, and for this purpose you only get limited help by knowing the reverse likelihood of what percentage each individual condition is to display the finding, because it would still be necessary to also know the epidemiology of each disease to calculate its likelihood.
Learning clinical pathology
For learning hematopathology, focus on the aspects of it that you may be expected to handle rather independently, mainly including peripheral blood smears and general screening of lymph nodes. Otherwise, a hematopathology workup is generally a very complex matter that should be performed by subspecialists thereof. In the unlikely event that you will end up at a workplace without an internal arrangement for how to consult a hematopathologist, make such arrangement yourself, since it will save you a lot of time compared to even trying to learn yourself how to perform a modern hematology workup.
For molecular pathology, focus on learning the situations in which molecular workup is needed, rather than the specific derangement. The latter can generally easily be looked up when needed, whereas the former can easily be missed.
Microbiology:
Put substantial effort on identifying what is actually memorization-worthy before starting to memorize microbiology-related content. For example, have an idea of the main workflows in a typical microbiology lab, but do not waste much time memorizing bench tests (negative/positive for indole, lactose/sucrose fermentation etc. etc.). In everyday practice there are only a limited number of bacteria that are identified by such testing, whereas the rest are generally identified by multitarget assays and/or MALDI-TOF. For exams where the passing score is an average of multiple topics (including the American Board of Pathology Certification Exams), focus on more useful topics instead.
General advice
- When making a mistake, admit that you did it and learn from it so as to focus on not repeating it. Also learn from the mistakes of others.
- Say "I don't know" instead of making up answers for what you do not know.
- Ask for help whenever needed, such as first time you are doing something, or whenever you are not sure about what to do, especially when doing something potentially irreversible. Also ask for help in moments whenever there is a high risk that you will not achieve what you need to do within a clinically acceptable time. Still, before asking, try to do as much as you can, as long as you do not do anything potentially irreversible, so that you can evaluate how you did it compared to the standard, and thereby know better how you will do it next time.
- Try to fit findings with the clinical picture so that the report makes sense, but do not make up findings that you do not see, especially in cases where you copy-paste words from a previous report or template. At the same time, do not omit relevant features just to fit an expected story.
- Do not wait for the whole pile. Whenever you can, do not be idle or do less urgent work while there is a pile of more urgent work gathering for you elsewhere. Instead, be familiar with where such piles are forming, and go there and grab whatever you may start working on right away.
- For larger specimens that need fixation before final grossing, you can still start writing a report of measurements and other externally visible findings to save time for later.
- Save your digital reports frequently.
- Focus on learning pitfalls and the interpretation of visual patterns and other non-written results, and do not waste much time memorizing information that can essentially always be conveniently and timely looked up when needed (but have an idea of where to find it). Further information: Learning pathology
Comprehensiveness
On this handbook, the following formatting is used for comprehensiveness:
- Minimal depth
- (Moderate depth)
- ((Comprehensive))
- << Decision needed between alternatives separated by / signs >>
- {{Common findings / In case of findings}}
- [[Comments]]
- Link to another page
- Organs, important regions or other important key words.
Emergent pathology
- REDIRECT Emergency pathology
Fixation
Immersion
Within an hour after removal from the body,[8] tissue samples should generally be placed in vessels with enough fixative to allow them to lie freely in the solution.[9] The standard fixation fluid is generally 10% neutral buffered formalin, which is roughly equivalent to 4% formaldehyde.[10] The ratio of tissue:formalin should be 1:5[11] to 1:10[12].[12]
Duration
The duration depends on tissue thickness, where formalin will penetrate and fix the tissue at ~1 mm/hour.[13]
When not to use formalin
The main exceptions to using formalin are mainly:
- Intraoperative consultation.
- Suspected crystals, such as a tophus or other specimen suspicious for gout versus pseudogout. These should be sent in alcohol or dry, since formalin will dissolve the crystals.
- Suspected lymphoproliferative disorders, such as lymph nodes (or other lymphoid aggregates) with a suspicion of lymphoma, where samples are generally put in a special solution for flow cytometry.
- Need for genetic testing, such as some cases of products of conception.
- Cytology specimens, which are preferably sent fresh (such as in red top tubes) to be processed within a few hours. If processing may be after a few hours, put tubes on ice, or add 50% alcohol.[14]
- Need for microbiology evaluation, mainly bacterial culture. For potentially infectious workups, check with the microbiology lab if they have the tissue they need before putting the specimen in formalin.
- Need for immunofluorescence, such as immune complex-mediated disease, where specific preservation will give better test sensitivity.[15]
If you don't know, and if you cannot soon get in touch with anyone who can guide you, specimens can generally be stored in a fridge in the meantime, even overnight if it is late (but make sure to follow-up as soon as possible in the morning). Until then, don't put the specimen in formalin and don't freeze the specimen.
Gross processing
Following are general notes on selection and trimming in pathology.
Priority
|
1. More invasive intraoperative consultations |
For prioritizing when you have more than one thing ongoing at the same time, the list at right can be used.
Fresh specimens
The initial processing of fresh specimens is also termed triaging.
- For intraoperative consults including frozen sectioning, see separate article on Emergent pathology.
When triaging a specimen, the measurements are most important as these may change after fixation. Other descriptions can generally be made after the specimen is fixed.
- See also separate article on fixation, including what specimens should not immediately be put in formalin (mostly tophus, lymph nodes and products of conception).
Before cutting
- Confirm that the patient identity on the specimen container matches the identity that will be applied to the gross description and cassettes. {{If the referral or requisition form is available, confirm the patient identity on that one as well.}}
- (Check for any discrepancy between the specimen description on the container and on the referral or requisition form, such as left versus right.)
- Don't omit any piece in the container, such as ones hidden in wraps.
- Generally measure in 3 dimensions, or in volume, but the greatest dimension is generally enough for specimens less than 0.4 cm.
- Generally weigh entire organs, after having any attached tissue trimmed away if feasible.
- (Note the color of the sample, even when unremarkable, but do not linger on deciding it.)[note 10]
- Generally, use inking for resection margins where tumor radicality is important. Further information: inking
- (On fatty or greasy surfaces, apply vinegar to emulsify and remove the fat, dry the specimen and then ink. Otherwise, vinegar can be used either before or after inking to "dry" it.)
- (Preferably photograph or make a drawing where slices have been taken.)[16]
- Remove any surgical stitches from samples before microtomy.
- (At least for larger samples, consider looking for medical imaging or biopsy reports in order to better guide the process.)[17]
- Fix bone in formalin prior to decalcification. Use reminders so not to forget bone that is decalcifying.
- Plan in which order to cut different parts so as to be able to take all relevant sections. Generally sample relevant surgical margins and smaller parts first, as these may be harder to find or even be fragmented later on.
Cutting
- When cutting with the longer knives, try to cut in one stroke - do not use like a saw (continuous back and forth)
- Generally, strive to make slices perpendicular to visible interfaces of relevant tissues.
- Generelly dissect and inspect the entire specimen, while keeping relevant parts intact enough for presentation to seniors and/or maintaining orientation.
- Trim tissues for microscopy examination to a thickness of maximum 3-4 mm.[note 11]
Perpendicular versus en face sections
Two major types of sections in gross processing are perpendicular and en face sections:
- Perpendicular sections allow for measurement of the distance between a lesion and the surgical margin.
- En face means that the section is tangential to the region of interest (such as a lesion) of a specimen. It does not in itself specify whether subsequent microtomy of the slice should be performed on the peripheral or proximal surface of the slice (the peripheral surface of an en face section is closer to being the true margin, whereas the proximal surface generally displays more area and therefore generally has greater sensitivity in showing pathology, also compared to perpendicular sections).
- A shaved section is a superficial en face slice that contains the entire surface of the segment.
Tissue selection
When sampling sections to submit for microscopic examination, whenever you sample from something that looks abnormal, generally try to also sample from the same type of tissue that looks normal.[note 12]
Biopsy wraps, bags and sponges
Put the following types of specimens in bags:
- Tiny specimens that need to be poured out from their containers.
- Bloody specimens such as endometrial curettages or products of conception. For products of conception, chorionic villi may otherwise contaminate other specimens. Bloody specimens may stick to wraps, so generally avoid that situation.
- Friable tissue such as urinary bladder biopsies.
Put the following types of specimens in bags, wraps or sponges:
- Other tiny specimens
- ((Any small piece of tissue where there is no leftover specimen to retake sections, since tissues occasionally get lost from cassettes, and the absence of a wrap, sponge or bag in the cassette of such cases points towards a mistake made at gross processing.))
Specimens must be fixed enough to be put on sponges.
H&E staining urgency
(Even in departments where other staff are primarily responsible for determining the urgency of H&E staining of each specimen, still double-check that it is correct if you can, such as by cassette color.) A major indication for rushing cases through H&E staining is a high risk of cancer, especially where immunohistochemistry staining will likely be performed, and the decision and types of staining will be determined by the standard H&E stain. Tissues that are generally rushed are:
- Brain biopsy.
- Lung biopsy.
- Breast needle biopsy.
- Biopsy from known tumor tissue.
- Suspected malignant lymph nodes, including lymphoma. However, these are generally not urgent when submitted together with a tumor, except mainly for the following (which are generally urgent):
- Pelvic sentinel lymph nodes
- Sentinel lymph nodes from known invasive lobular carcinoma (but not invasive ductal carcinoma)
In both these cases, the cases are rushed so that immunohistochemistry can be performed if a metastasis is not readily detected on standard H&E slides, so that it is available by the time the rest of the slides are out. Immunohistochemistry in these cases can detect micrometastases that are not readily visible on H&E stain, but are evident on cytokeratin AE1/AE3. However, if the lab stains such cases regardless of whether H&E stain shows a metastasis or not, then they do not need to be rushed.
Marking cassettes
Use only hard pencil (or specially purchased histology markers), as marks made with pens, Sharpie markers, or scientific freezer-safe markers can get dissolved in tissue processing.[18]
Evaluation
First, confirm that the identity of the sample evaluated is the same as that of the requisition form and/or other medical history.
Microscopy settings
Generally the condenser is placed in its highest position or just slightly lower. At low magnification objectives (mainly 4x and 10x objectives), the opening of the condenser (or iris) diaphragm should be wide open. This corresponds to turning away or "lowering" the condenser on microscopes where the condenser apparatus can be turned to the side (and is shown as "without condenser" in images below). For high-dry (40x) and oil-immersion objectives (100x), the diaphragm should be closed slowly while looking at a sharply focused section until the level of illumination is just slightly reduced, in order to attain optimal contrast and resolution (and corresponds to "with condenser" in images below).[19]
Low magnification has a greater span of focus compared to high magnification, so it is normal to need to focus if you're increasing magnification. If there's a constant visual artifact, even after you've cleaned the eye piece and objective lenses with lens tissue, try raising or lowering the condenser if you can, and the artifact may disappear out of focus.
Priority
Whenever you have more than one case, generally have an initial look at the requisition form and/or in the microscope at the most likely relevant area of each case, and prioritize the case(s) that likely need to be done first. Indications to prioritize case(s) are mainly:
- Clinical management of the patient highly benefits from a fast diagnosis.
- Being marked as a rush case.
- There's a significant probability that it will require further workup such as immunohistochemistry, especially when there is an impending deadline for ordering it.
- The optimal person to ask for advice may not be available later. Further information: Consultation
As part of initial triaging, also determine what tasks can be 'delegated to juniors or pathologist assistants.
For a pile of many cases of relatively low risk of a need to prioritize any one over another, such as gastrointestinal biopsies, a very quick glance at the forms and/or a naked eye look at the glass slides is generally sufficient.
Main steps
- Preferably, look up past medical history of the patient, mainly past cancers that could possibly appear in the current specimen.
- Try to pick up glass slides without touching the tissue area, as fingerprints may interfere with the evaluation.
- Look at each microscopy slide by plain eye, to plan the microscopy scanning so as to not miss peripheral fragments.
- Have a systematic direction of scanning through microscopy slides, such as from top left to bottom right as seen in the microscope. When the microscope makes what you see two-way mirrored, the starting position is with the objective pointing at the bottom right of the glass slide. You may center on findings on interest and evaluate them at higher magnification, and then resume the scanning
This scanning technique above makes sure that you don't miss any area, and also minimizes redundant scanning. The width or clock position that you scan in each field of view is not exact, as you may scan up to the entire width in cases where you think relevant findings will clearly present themselves even if only partially showing in the periphery of your view.
- Look in particular for whatever is requested or suspected on the requisition form or equivalent. For each type of condition, initially you will generally focus relatively more on high magnification features with high specificity, but you should still have a habit of looking at low magnification as well to get an idea of its pattern. In time, you will increasingly correlate diseases and conditions with their overall low magnification patterns - patterns that may require 1000 words to describe and thus cannot conveniently be part of written criteria, but will nevertheless allow you to make quicker and more accurate diagnoses, or at least clues thereof.
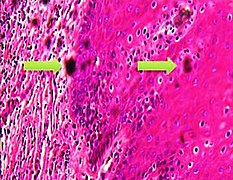
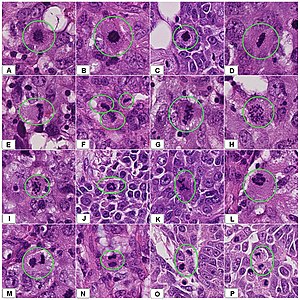
- When you find something suspicious, it is helpful at least in the beginning to evaluate them systematically by a low-to-high magnification approach (example below showing a basal-cell carcinoma):
- Complete the scanning even if you encounter a finding, as there may be additional findings as well. It is usually helpful to Google multiple images of the normal histology of the location you are looking at, and compare those to what you see, and focus on whatever features may deviate from the normal.
General patterns
Following are major patterns that often help in making a diagnosis.
Architectural patterns
Cellular patterns
Nuclear patterns
When feasible, classify nuclei as follows:
Pleomorphic when having different sizes and shapes. This often correlates with an increased nucleus to cytoplasm ratio. These features generally favor malignancy in the evaluation of suspected malignancies.
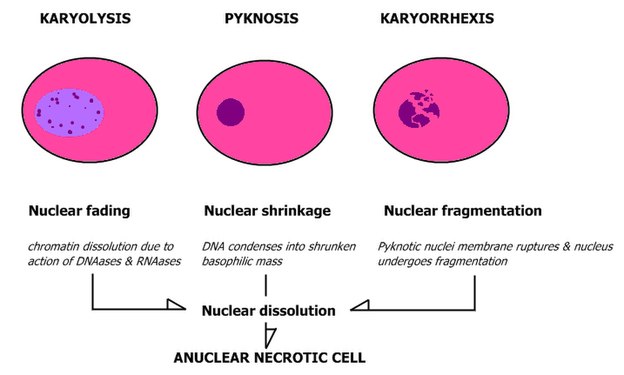
- Patterns of nuclear disintegration
Other patterns
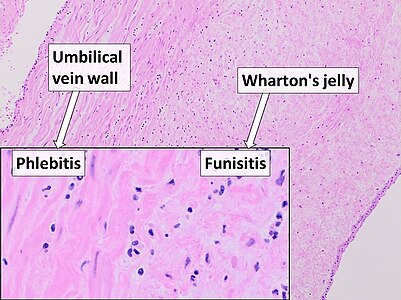
Inflammation
Marking slides
...Then slowly approach the slide while making sure that the tip remains in the location you want to mark. Be careful not to obscure findings of interest with the mark. If the slide is likely to undergo whole slide imaging, make the mark on the back and not on the coverslip (as it reduces the risk of erroneous focusing of the cameras).
Measuring distances
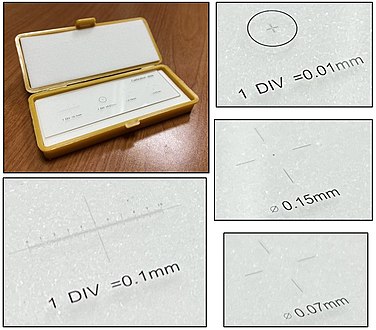
There are also eye pieces that show a ruler in the field of view, but make sure you match it with the correct objective and other settings to make the measurement valid. A calibration slide is more robust because of such sources of error.
Counts per mm2
There are multiple situations where a finding will be quantified in terms of amount per mm2. To make such calculations, you need to know the size of the area you see in the microscope. It is usually possible to look up what theoretically would be the area, but the most reliable way of knowing is to use a calibration slide to measure the diameter of your view. The area is then calculated as:
- Area in mm2 ≈ (diameter in mm2) x 0.79
On the imaged example, each square is 0.05 mm wide, and each line represents 0.01 mm, making the diameter of the field of view 0.55 mm in this case. Thus, you can calculate the area in mm2 by Googling:
- 0.55 x 0.55 x 0.79
- (equals approximately 0.24 mm2).
Generally count at least 10 fields of a high power view (or more if specifically instructed). You can keep the count in your head by repeating the total count and the order of the area you are looking at, such as:
- "Zero (instances) of one (field of view)"
- "One of two (as you see one instance as you've moved to the second field of view)"
- "Two of two" etc.
Subsequently, the count per mm2 is calculated as follows:
| Count per mm2 = | Total count |
| Number of fields x Area per field (in mm2) |
For example, if you reached "15 of 10", and the area of your field is 0.2 mm2, the count per mm2 can be calculated by Googling:
- 15/10/0.2
- = 7.5
Sometimes "high power field" (HPF) is used for area, but it has a substantially different area for different microscopes, for example:
| Microscope type | Area per HPF |
|---|---|
|
0.096 mm2 [20] |
| AO with 10x eyepiece | 0.12 mm2 [20] |
| Nikon Eclipse E400 with 10x eyepiece and 40x objective | 0.25 mm2 |
| Leitz Ortholux | 0.27 mm2 [20] |
| Leitz Diaplan | 0.31 mm2 [20] |
When your instructions are to count a specific number of HPFs, one HPF can be assumed to be 0.2 mm2.[21] If the view area in your microscope significantly differs from this area, calculate how many views you need to count as:
| Views = HPFs required x | 0.2 |
| Your microscope area (in mm2) |
For example, if your instruction is to count 10 HPFs and each view in your microscope shows 0.096 mm2, you should count in this many views:
| 10 x | 0.2 | ≈ 21 |
| 0.096 |
Subsequently, if your microscope area is significantly different from 0.2 mm2 and you need to state your result in terms of count/HPF, use:
| Count/HPF = Average count in your view x | 0.2 |
| Area of your view in mm2 |
For example, if you have counted an average of 10 cells (or other object of interest) in each of your views, and the area of your view is 0.096 mm2, then your count/HPF is:
| 10 x | 0.2 | ≈ 21 |
| 0.096 |
Artifacts
In microscopy, an artifact is an apparent structural detail that is caused by the processing of the specimen and is thus not a legitimate feature of the specimen. Major artifacts to account for include:
Differential diagnoses of artifacts are mainly:
- Foreign bodies. In contrast to contamination, these conform more naturally to the surrounding tissue.
- Organisms, to be particularly considered when there are multiple objects of the same size.
Order recuts from the same paraffin-embedded tissue if artifacts significantly impairs your diagnostic evaluation of the glass slide. However, artifacts caused by gross processing may affect recuts as well.
Micrography and telepathology
Unless you have more specific equipment for taking microscopic images and showing cases to remote colleagues, you can perform these tasks as follows:
- With a stationary computer, you can connect to a microscopy camera. For telepathology, you can start a videoconferencing session with your senior, then share the screen while showing a micrograph, or the live view so that you can move around.
- With a mobile phone:
- You can send micrographs to your senior, but it is technically difficult to keep the focus while moving the glass slide.
Consideration
Consider the adequacy of the specimen. Have a somewhat lower threshold to make a report of "inadequate" or "insufficient" if it is easy to resample from the patient, such as remaining diagnostic tissue at a superficial location.
First, generally suspect the common conditions for the location at hand. A less common variant of a common condition is often still more likely than a rare condition, so generally call the latter only if it really fits the picture.
Whenever you consider a certain diagnosis, also consider whether it is one step better or worse. For example, when you consider a non-invasive but high-grade dysplasia, also consider both low-grade dysplasia and invasiveness. </noinclude>
|
Further reading: |
Notes
- ↑ Further information on what is memorization-worthy or not: Learning pathology
- ↑ The printable version URL is: http://patholines.org/index.php?title=Starting_pathology&printable=yes
The recommended letter format version in Firefox is scale 100%, or Google Chrome scale 135%. To save it for offline use, choose to print it as a PDF file. Google Chrome gives you a smaller file size than Firefox (about 30 MB compared to about 240 MB in Firefox, but also slightly less image quality). - ↑ As a metaphor, pathologists should not waste time memorizing "elephants in the room", which will most definitely be spotted, and if you don't know which subtype, you will most definitely look it up or ask an expert if needed. Rather, pathologists should memorize how to spot "chameleons in the corner", which may not be spotted if not knowing what to look for.
- ↑ If you worry about Internet outages, then it is still much more worthwhile to purchase satellite Internet as a backup (which costs $50 to $100 per month) than to try to memorize the Internet.
- ↑ The author has no financial or other conflict of interest in the mentioning of ImmunoQuery.
- ↑ Further information on what is memorization-worthy or not: Learning pathology
- ↑ Further information on what is memorization-worthy or not: Learning pathology
- ↑ As of July 2023, PathPrimer has 2270 Multiple Choice Questions (and has the highest quality of its questions despite an overall bad image quality), PathDojo has 1700, BoardVitals has 1050, and the ASCP Question Bank has 675 MCQs. AceMyPath has multiple whole slide images, but is not really a Qbank.
- ↑ The highest yield questions are generally those with one teaching point per question. In contrast, for a true/false question to be of decent quality, each alternative should be relevant as per criteria above, but it is more difficult to create than low-quality ones. Since low-quality true/false questions are generated at much higher rate, the vast majority of true/false questions are subsequently low-quality.
- ↑ The color of gross specimens generally has very limited clinical significance.
- ↑ Thicker slices may not become adequately fixated in formalin.
- ↑ Normal sections from the same tissue helps identifying what is histologically abnormal in the grossly abnormal tissue, versus normal individual variations.
Main page
References
- ↑ . Copyright Law OF THE United States and Related Laws Contained in Title 17 of the United States Code. U.S. Copyright Office. May 2021
- ↑ . 17 U.S. Code § 102 - Subject matter of copyright: In general. Cornell Law School, Legal Information Institute. Retrieved on 2022-01-11.
- ↑ . Copyrightability of Tables, Charts and Graphs. Deep Blue Repositories, University of Michigan Library. Retrieved on 2022-01-07.
- ↑ Image by Mikael Häggström, MD, using source images by various authors. Source for useful context in problem-based learning: Mark A Albanese, Laura C Dast (2013-10-22). Understanding Medical Education - Problem-based learning. Wiley Online Library.
- ↑ Kräenbring, Jona; Monzon Penza, Tika; Gutmann, Joanna; Muehlich, Susanne; Zolk, Oliver; Wojnowski, Leszek; Maas, Renke; Engelhardt, Stefan; et al. (September 24, 2014). "Accuracy and Completeness of Drug Information in Wikipedia: A Comparison with Standard Textbooks of Pharmacology ". PLOS ONE 9 (9): e106930. doi:. PMID 25250889. Bibcode: 2014PLoSO...9j6930K.
- ↑ Ellen D’Hooghe, M.D., Gordan M. Vujanic, M.D., Ph.D.. Kidney tumor - Childhood tumors - Nephroblastoma. Pathology Outlines. Topic Completed: 16 September 2020. Minor changes: 6 October 2021
- ↑ Shaofeng Yan, M.D., Ph.D.. Skin nonmelanocytic tumor, Carcinoma (nonadnexal), Squamous cell carcinoma. Pathology Outlines. Topic Completed: 13 January 2020. Minor changes: 8 December 2021
- ↑ . Breast pathology grossing guidelines. UCLA Health. Retrieved on 2021-09-09.
- ↑ Katarzyna Lundmark, Krynitz, Ismini Vassilaki, Lena Mölne, Annika Ternesten Bratel. Handläggning av hudprover – provtagningsanvisningar, utskärningsprinciper och snittning (Handling of skin samples - Instructions for sampling, cutting and incision. KVAST (Swedish Society of Pathology). Retrieved on 2019-09-09.
- ↑ . Paraformaldehyde, Formadehyde and Formalin. Duke University. Retrieved on 2019-12-17.
- ↑ . Fixation of Tissues. Approval Date: August 2016, August 2020. Review Date: August 2024|website=Royal College of Pathologists of Australia
- ↑ 12.0 12.1 Buesa RJ, Peshkov MV (2012). "How much formalin is enough to fix tissues? ". Ann Diagn Pathol 16 (3): 202-9. doi:. PMID 22483550. Archived from the original. .
- ↑ . How to Submit Tissues for Embedding. University of Pittsburgh, Starzl Transplantation Institute. Revised 04/19/21
- ↑ . How to send fluid and make good cytology slides. Tufts University.
- ↑ Mubarak M, Kazi Javed I, Kulsoom U, Ishaque M (2012). "Detection of immunoglobulins and complement components in formalin fixed and paraffin embedded renal biopsy material by immunoflourescence technique. ". J Nephropathol 1 (2): 91-100. doi:. PMID 24475396. PMC: 3886135. Archived from the original. .
- ↑ Monika Roychowdhury. Grossing (histologic sampling) of breast lesions. Pathologyoutlines.com. Topic Completed: 1 August 2012. Revised: 19 September 2019
- ↑ . Gross Pathology Manual By The University of Chicago Department of Pathology. Updated 2-14-19 NAC.
- ↑ . Histopathology Services. UNC School of Medicine. Retrieved on 2021-11-15.
- ↑ Patrice F Spitalnik. Histology Laboratory Manual, Vagelos College of Physicians & Surgeons Columbia University. Retrieved on 2021-09-20.
- ↑ 20.0 20.1 20.2 20.3 . Infiltrating Ductal Carcinoma of the Breast (Carcinoma of No Special Type). Stanford University School of Medicine. Retrieved on 2019-10-02.
- ↑ Klimstra, David S.; Modlin, Irvin R.; Coppola, Domenico; Lloyd, Ricardo V.; Suster, Saul (2010). "The Pathologic Classification of Neuroendocrine Tumors ". Pancreas 39 (6): 707–712. doi:. ISSN 0885-3177.
- ↑ 22.0 22.1 22.2 Taqi, SyedAhmed; Sami, SyedAbdus; Sami, LateefBegum; Zaki, SyedAhmed (2018). "A review of artifacts in histopathology ". Journal of Oral and Maxillofacial Pathology 22 (2): 279. doi:. ISSN 0973-029X.
Image sources
</noinclude>
Cytology introduction
Overall evaluation
In addition to a general evaluation, also evaluate the following in cytology samples, or any sample with scattered cells rather than coherent tissue:
- Consider looking for any additional concurrent samples from the same patient, especially excisions, as usually correlate.
- Adequacy of specimen. Cells may be too few or too obscured by other material to make a proper diagnosis.
- Background, mainly if it is clear or dirty
- Overall cellularity
- Artifacts
Tumors, introduction
Gross processing
Gross tumors according to organ and/or tumor type (as per above) whenever possible. In additional to general gross processing guidelines, the following instructions are usually acceptable:
- Identify surgical margins where tumor involvement may be necessary to identify or negate.
- Generally ink such margins.
- Section the specimen so as to get a gross overview of tumor extent.
- (Take a gross photograph of the tumor.)
- Describe the tumor, minimally by color and consistency (firm/rubbery versus semisolid), and possibly also including diffuse versus well-demarcated.
- Measure tumor dimensions and distances to relevant margins.
- Sampling of generally at least one slice per centimeter of tumor (which for larger tumors may be submitted as 2-3 sections per cassette).
Further information: Gross processing
By gross appearance
Evaluation
The most important aspects of a tumor is whether it is malignant or not, and staging.
Evaluation of suspected malignancies
For evaluation of suspected malignancies such as tumors, the most important aspect is whether it is benign or malignant. If malignant, then staging is necessary.[3] There are generally specific criteria for various forms of tumors, which should be used whenever applicable, but following are some generalizations.
A general approach is to start looking at a slide which seems to contain non-necrotic tumor, and if possible it should also show surrounding non-tumor tissue, so that the interface can be appreciated (and tumors are generally less necrotic at the periphery).
Benign or malignant
| Benign[4] | Malignant[4] | |
|---|---|---|
| Gross examination |
|
Possibly:
|
| Microscopy | Almost no irregularities of cellular structures | Nuclear atypia:
|
Primary tumor versus metastasis

Indications of a metastasis rather than primary tumor are mainly:
- Tumors that are unlikely to arise at the location at hand.
- Tumors conforming to more likely metastasis pathways.
If a suspected malignancy is present, generally check the patient history for any history of cancer, especially for tumors in more common metastasis sites, which mainly include lung, bone, liver and/or brain. In case of such history, preferably look at the microscopy slides of the past cancer to help determining whether the current case is of the same origin, versus a primary at the current body location, versus a metastasis of yet another location. If there is no known history of cancer, still consider a metastasis of unknown primary origin, especially for suspected malignancies in lymph nodes, liver, lungs, bones, or skin.[6]
Further information: Metastasis
Histopathologic type
For specific diagnoses by organ system, see anatomic diagram on Patholines Main page. This resource will give the main steps towards reaching a diagnosis, but before making a tumor diagnosis, generally be sure that it fulfills the criteria of the condition according to The WHO Classification of tumors, and generally consult an experienced pathologist as well until you feel confident.
Visually, tumors and other suspected malignancies can usually be classified into one of the following groups:
Squamoid tumors, typically having abundant eosinophilic cytoplasm.[7]
Further pinpointing of a specific tumor type is often attained by thinking of one or more possible diagnoses, and looking up their differential diagnoses, followed by comparing their microscopic descriptions and multiple micrographs with the case at hand. When two or more diagnoses seem to fit with the case at hand, consider performing immunohistochemistry. Find relevant target proteins that are expected to stain substantially differently between the possible diagnoses. If it's not evident from initial sources, you may use Immunoquery.com which will generally suggest the most relevant target proteins to distinguish the suspected conditions at hand.
Gland-like tumors
Gland-like tumors are mainly evaluated for cellular atypia, architectural dysplasia and invasion, and thereby classified into the following main categories:
- Hyperplastic lesions, lacking significant atypia
- Adenomas, which can range from mild to high-grade dysplastic, yet are generally confined within their anatomic layers, that is, they are not invasive.
- Adenocarcinomas, with the main criterion being invasiveness. Evaluate specifically by location when possible. Some specific locations included in this resource:

Generally, adenocarcinomas are categorized by degree of differentiation as follows:
- Well differentiated: > 95% of tumor has glandular formations
- Moderately differentiated: 50 - 95% glandular
- Poorly differentiated: < 50% glandular
If metastatic adenocarcinoma seems to be the case (always consider this in at least lung adenocarcinoma), look at the history and radiology reports for a most likely primary. Generally, perform immunohistochemistry with one or two stains that are most typical for the suspected primary, and optionally add CK7 and CK20 as a broad screening. Further information: Metastasis
Squamoid tumors
These are more or less looking like a squamous-cell carcinoma:
Differential diagnoses depend on location, such as:
- Squamous-cell carcinoma of the skin, with multiple differential diagnoses
- Squamous-cell carcinoma of the lung
- Urinary bladder: Urothelial versus squamous-cell carcinoma
- Cervix uteri: Cervical dysplasia
Spindle-cell tumors
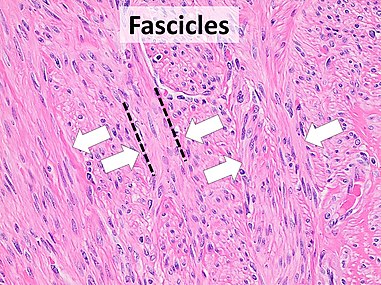
For Spindle-cell tumors, the shape of the nuclei is a clue to the diagnosis, with the following tendency:
- Pointed on both ends: True fibroblastic tumors
- Pointed on one end and blunted on the other ("bullet-shaped"): Neural
- Blunted on both ends ("cigar-shaped"): Smooth muscle
- Triangular: Myofibroblastic
Evaluate specifically by location when possible
Further histopathologic subtyping and grading
Beyond determining overall malignancy diagnosis (such as adenocarcinoma), probable origin and staging, classification of tumors into a specific histopathologic type or grade is generally of relatively less value. In cases of clearly non-malignant tumors where it is difficult to determine the specific histopathologic type or grade, it is generally acceptable to conclude the evaluation and report it as such, unless the clinician specifically requests otherwise. For potentially malignant or high-risk tumors, typing and grading often still affects the management, but generally much less so than staging.
Undifferentiated malignancy
An initial panel of cytokeratin (CK, such as by CK AE1/AE3 cocktail), S100, vimentin and LCA (CD45) can be used (see source article for subsequent work-up).[8]
Alternatively, a more comprehensive panel can be performed upfront, with the most pertinent panel suggested below (with main associated primary origins in parentheses), but it should be tailored to additional clues from each individual case:[9]
- Mucicarmine or Cytokeratin CAM 5.2 (adenocarcinoma)
- CK7 and CK20 can give a broad indication of the primary site. Still use more specific immunohistochemistry stains instead or in addition where applicable. See table below for main patterns.
- NKX3.1 in males (prostate adenocarcinoma), or PAX-8 or estrogen and progesterone receptor in females (female reproductive tract).
- TTF-1 (thyroid or lung tumor)
- GATA3 (urothelial carcinoma, breast tumor)
- CDX2 (gastrointestinal tumor)
- p63 (squamous cell carcinoma)
- S100 (neural, invasive melanoma)
- LCA (CD45) (hematopoietic)
- Synaptophysin (neuroendocrine)
| CK20 | |||
|---|---|---|---|
| Positive | Negative | ||
| CK7 | Positive |
|
|
| Negative |
|
| |
Non-neoplastic
If a neoplasm has been ruled out for what clinically appeared like a tumor, seek a diagnosis that can be consistent with the clinical findings that caused the suspicion. If no explanation is found on the slides, generally take additional levels on the paraffin block, or more sections from any leftover tissue.
For example, for a breast biopsy of what appeared to look like a mass, and there is no neoplasia, look mainly for dense fibrosis or other fibrous changes, so that you can report it and thereby explain the finding, rather than merely writing "benign breast tissue".
Heterogeneity
After having characterized a suspected malignancy, still screen through it for any significant areas that are different and may need own mentioning, or even change the overall type or grade.
Additional levels or slices
Situations requiring additional material include mainly where tumor is expected but nevertheless not seen on existing slides. Such cases include:
- The gross report or other observation describes a tumor or polyp, but none is seen on microscopy.
- Re-excision does not identify tumor cells in a clearly non-radical primary excision or biopsy.
Also consider more material if the most aggressive pattern is seen in the last available section, in which case more sections are indicated (from the same paraffin block if additional tissue is not available).
Depending on availability and greatest suspicion, additional material is either acquired by taking addition step sections of remaining tissue in a paraffin block, or taking additional slices from the original specimen.
Staging
Staging is generally done by TNM classification. Specific TNM systems should be used whenever applicable, mainly the manual by the American Joint Committee on Cancer (AJCC) if you can access it. Further information: Secrets Otherwise, a general system may be used:[3]
|
T: size or direct extent of the primary tumor
N: degree of spread to regional lymph nodes
M: presence of distant metastasis Further information: Metastasis
|
Put your main focus on features that will determine the final stage. For example, if you see a lymph node involved by cancer, the presence or absence of lymphatic invasion is no longer critical, but rather the presence or absence of additional involved nodes or distant metastasis. If a stage-defining finding is uncertain, generally fall back to the lower stage.
For the size, the greatest dimension of the tumor is most important. Second and third dimensions (preferably at right angles compared to each other) are optional. Use the largest measure for each dimension. For example if one slide shows a tumor measuring 2.0 x 1.0 cm, and another slide shows the same tumor measuring 1.5 x 1.5 cm, then the tumor can be reported as measuring 2.0 x 1.5 cm. The third dimension can be estimated from gross measurement, or, if the tumor is entirely submitted, adding together the presumed thickness of each slice where tumor is found microscopically. For excisions with less than a centimeter of tumor, and where there has been a previous biopsy, consider looking at the size of tumor on the previous biopsy, which may be the largest measurement, and therefore the measurement of choice for staging.
Nx: lymph nodes cannot be assessed also applies when you only find lymph nodes that are not within the physiologic drainage path of the cancer, and they are benign.
Radicality

Determine if malignant cells are located close to, or even in, any surgical resection margins.
Lymphovascular invasion
Lymphovascular invasion should always be mentioned. When present at margins, it does not count as tumor extension.
Molecular workup
Ensure that any cancer undergoes reflex testing where applicable by local guidelines. When they are not applicable, a rule of thumb is to order individual genetic testing if there is a need to test up to 2 genetic targets, and to order genomic sequencing if more targets need genetic testing (not counting immunohistochemitry or other more easily performed tests).
When needing to send out tissue to an outside laboratory, follow their requirements and guidelines for each test. Generally, choose the tissue block with the greatest amount/area of the highest grade malignancy. For molecular proliferation tests (other than immunohistochemistry) such as Ki67, avoid samples with previous biopsy site or inflammation (as these will cause a false high proliferation rates). On the other hand, for next-generation sequencing, hemorrhage, necrosis and adipose tissue does not need to be minimized, as these contain only little genetic material.
Reporting
For cancers, generally include a synoptic report, such as per College of American Pathologists (CAP) protocols at cap.org/protocols-and-guidelines. If there is no CAP synoptic available for the cancer type at hand, attempt to find one in the latest AJCC Cancer Staging Manual (see the Secrets article), but make sure to use pathologic staging (with a p preceding the T, N or M) rather than clinical staging.
If a surgery produces a specimen with cancer, as well as re-excisions from certain directions, you should preferably give the closest distance to margins in each specimen, as well as the closest distance overall in a synoptic, for example:
A. (Specimen with most of the cancer)
B. (Re-excision in the direction of the medial margin)
Synoptic report
|
If possible, generally include features of any previous biopsies or smaller excisions in synoptic reports and staging. For example, if staging is based on tumor size, and the tumor size in a previous excisional biopsy corresponds to a higher stage than the size of residual tumor in a subsequent wider excision, then the report of the latter should give the higher stage, with a comment thereof, such as:
- pT__
- - Based on previous excisional biopsy (specimen ID: ______)
See also: General notes on reporting
Metastasis
Gross processing
For suspected tumors in bone or other tissue that generally needs decalcification, try to grossly separate some suspicious soft tissue to be processed without decalcification, as decalcification might affect any molecular studies that will later be indicated. See site-specific grossing guidelines for further directions.
Metastasis of unknown primary
| Histopathologic type - see section below for descriptions[12] |
Relative incidence among metastases of unknown primary origin[12] |
| Well and poorly differentiated adenocarcinomas | 50% |
| Undifferentiated carcinoma | 30% |
| Squamous cell carcinoma | 15% |
| Undifferentiated neoplasms | 5% |
- Memorization-worthy:[note 1] Do not diagnose a renal cell carcinoma metastasis without radiologic evidence of a renal tumor. To be a plausible primary tumor for a renal cell carcinoma metastasis, a renal tumor should be visible radiologically. In cases of suspected renal cell carcinoma but no renal imaging is available, it is reasonable to ask the ordering doctor to allow you to wait with signing out the pathology report until renal radiology has been performed.
Undifferentiated malignancy
An initial panel of cytokeratin (CK, such as by CK AE1/AE3 cocktail), S100, vimentin and LCA (CD45) can be used (see source article for subsequent work-up).[8]
Alternatively, a more comprehensive panel can be performed upfront, with the most pertinent panel suggested below (with main associated primary origins in parentheses), but it should be tailored to additional clues from each individual case:[13]
- Mucicarmine or Cytokeratin CAM 5.2 (adenocarcinoma)
- CK7 and CK20 can give a broad indication of the primary site. Still use more specific immunohistochemistry stains instead or in addition where applicable. See table below for main patterns.
- NKX3.1 in males (prostate adenocarcinoma), or PAX-8 or estrogen and progesterone receptor in females (female reproductive tract).
- TTF-1 (thyroid or lung tumor)
- GATA3 (urothelial carcinoma, breast tumor)
- CDX2 (gastrointestinal tumor)
- p63 (squamous cell carcinoma)
- S100 (neural, invasive melanoma)
- LCA (CD45) (hematopoietic)
- Synaptophysin (neuroendocrine)
| CK20 | |||
|---|---|---|---|
| Positive | Negative | ||
| CK7 | Positive |
|
|
| Negative |
|
| |
Metastatic adenocarcinoma
If metastatic adenocarcinoma seems to be the case (always consider this in at least lung adenocarcinoma), look at the history and radiology reports for a most likely primary. Generally, perform immunohistochemistry with one or two stains that are most typical for the suspected primary, and optionally add CK7 and CK20 as a broad screening.
Generally, classify by degree of differentiation as follows:
- Well differentiated: > 95% of tumor has glandular formations
- Moderately differentiated: 50 - 95% glandular
- Poorly differentiated: < 50% glandular

Example report:
A. Right lung mass, CT-guided needle biopsy:
Comment: Immunohistochemistry was performed, and showed tumor cells positive for CDX2 and CK 20, and negative for CK 7, consistent with metastasis from a gastrointestinal primary tumor. |
Further workup
Perform additional biomarker testing as per local guidelines for the corresponding primary tumor at metastatic/advanced stage (such as mismatch repair proteins and next generation sequencing in case of metastatic colon adenocarcinoma).
Immunohistochemistry
When learning pathology, the percentages by which immunohistochemistry (IHC) results are positive or negative for various diseases are generally easily looked up when needed, so what a pathologist needs to learn is mainly how to select the optimal immunohistochemistry panels in the first place for various presentations where the diagnosis is unknown.
Immunohistochemistry ordering
The main approaches to immunohistochemistry ordering are:
- If there is insufficient tissue left for immunohistochemistry after standard (usually H&E) staining, you can potentially ask a histotechnologist to destain a glass slide and subsequently perform IHC on that slide. For small specimens, if you predict that there will be several stainsin the near future, order extra unstained slides upfront (since there is always a bit of a waste of tissue at the microtome if the paraffin block needs to be remounted and cut later).
- Look for a specified panel for the presentation at hand. For example, for an undifferentiated tumor with no clear lineage differentiation, an initial panel of cytokeratin (CK), S100, vimentin and LCA (CD45) can be used.[8] Further information: Tumor evaluation
In cancer cases, also be familiar with local routines for reflex tests by various cancer types, which may include both immunohistochemistry and other molecular tests. - Coming up with the most relevant differential diagnoses for the case at hand, and find the immunohistochemistry stains that best distinguish them. Immunohistochemistry profiles for diseases and conditions, as well as their main differential diagnoses, is generally found at Pathology Outlines, or you can pay for a subscription to ImmunoQuery[note 2]:
Using Immunoquery
The main page of ImmunoQuery gives you the "Diagnoses" option, where you enter up to 3 differential diagnoses to generate the optimal immunohistochemistry panel to differentiate them (you may need to click ∨ Suggested Panel to show it if it is collapsed). It also displays an automatic message when the included antibodies/immunostains are not sufficient for a satisfactory panel, in which case you can:
- Make a specific search only including the two conditions where suggested immunostains were insufficient, if you had previously compared 3 diagnoses.
- Consider additional stains from the "Comprehensive panel" displayed below the suggested one.
- Switch the "Sensitivity" setting (seen at top) from 1 (which means that diffuse, focal as well as not specified staining count as positive) to 3 (which means that only diffuse staining counts as positive whereas both focal and absent staining count as negative, and references without any specified staining pattern are omitted from the analysis). This has less data to support the suggested stains (since many references do not specify whether positivity was diffuse or focal), but can sometimes state a better distinction between conditions. When including a stain based on its distinguishing features on a sensitivity setting of 3, you need to keep the practice of classifying only diffuse staining counts as positive, and focal to absent staining as negative.
Test question
The attending gives you a lung biopsy case to preview. You are first uncertain about the type of tumor, so you ask a fellow resident, who finds a diagnostic area of the tumor and tells you that this is a typical squamous cell carcinoma. You also look through the patient's history, and find that the patient has had a squamous cell carcinoma of the anus in the past, and you now want to find out whether the tumor originated in the lung, or if it is a metastasis from the anus, or possibly the skin. You therefore do an ImmunoQuery lookup, with the following results:
Insufficient antibodies for a satisfactory panel to differentiate Lung squamous cell carcinoma and Anus squamous cell carcinoma
You go talk with the attending, who agrees that EpCAM, GATA3 and p16 should be in the panel, but just as ImmunoQuery also tells, the attending thinks that the panel is not satisfactory to differentiate lung SCC from anus SCC, and wants you to add one more stain to improve the panel. You go back to ImmunoQuery and increase the Sensitivity from 1 to 3 (which means that only diffuse staining counts as positive whereas both focal and absent staining count as negative, and references without any specified staining pattern are omitted from the analysis), and get the following results:
You also perform a repeated search by only entering Lung and Anus SCC, and you get the following results:
Top results:
You switch sensitivity from 1 to 3 for this result as well, showing:
No antibodies to differentiate Lung squamous cell carcinoma and Anus squamous cell carcinoma
Top result:
You go talk with a technician at the histology lab, and your hospital offers all the stains in the alternatives, at similar costs, so you don't have to think about expenses and logistics of sending the case out to external labs. In addition to EpCAM, GATA3 and p16, what is the best alternative, given the information you retrieved above, to differentiate lung versus anus squamous cell carcinoma?
GRPR is the top result when specifically comparing lung versus anus squamous cell carcinoma, both at sensitivity settings 1 and 3, with no major difference between them, and thus anus origin is favored in case of either diffuse or focal cytoplasmic staining. DLK showed as N/A for anus SCC. |
Chromogen color
Request a red rather than brown chromogen in heavily pigmented lesions, such as in some melanomas.
Evaluation
First be familiar with which cells on the slide are being evaluated, such as first looking at a slide with standard staining (usually H&E) to avoid counting background cells.
When the relevant cells take up stains, confirm whether their staining pattern actually counts as positive. For example, a cytoplasmic staining for p63 still counts as "negative (cytoplasmic)" when suspecting squamous, myoepithelial and prostatic basal cells (where only nuclear stain counts as positive). On the other hand, the same cytoplasmic staining for p63 counts as "positive (cytoplasmic)" when suspecting muscle differentiation.[15]
Interpretation
The main methods for interpreting immunohistochemistry results are:
- Looking up each differential diagnosis at for example Pathology Outlines and comparing their expected staining to see which entity is most likely.
- Paying for a subscription to ImmunoQuery,[note 2] where you can enter immunohistochemistry results and generate a list of most likely conditions with that profile.
Preferably, immunohistochemistry results will be very specific or sensitive for a suspected condition, thereby confirming it if positive, or excluding it if negative, respectively. Even when that is not the case, immunohistochemistry can at least alter the likelihoods of different differential diagnoses. In practice, clinicians or pathologists do not state exact or even approximate numbers of likelihoods of differential diagnoses (Further information: Reporting see the Reporting chapter for phrasing uncertainty), since reality is too complex for that, but to demonstrate the general principle of how immunohistochemistry results can be calculated, the following formula can be used:
- Gross likelihood of a disease/condition = (Pre-test probability) x (Probability that the condition shows the immunohistochemistry results at hand).
The pre-test probability is a product of for example the incidence of the condition in the patient's epidemiologic type such as age and sex, as well as the probability that the condition would have caused the clinical course, including signs and symptoms, as well as the microscopic impression. For example, if you want to differentiate a pleomorphic liposarcoma from a pleomorphic rhabdomyosarcoma in soft tissue, you may find in ImmunoQuery that the following stains are most efficient in distinguishing the two, with the following percentages of being positive:
| Soft tissue pleomorphic liposarcoma | Soft tissue pleomorphic rhabdomyosarcoma | |
| Desmin | 17% | 95% |
| Actin HHF-35 | 0% | 71% |
Let's say for example that your pre-test probability was about 30% for pleomorphic liposarcoma and 70% for pleomorphic rhabdomyosarcoma, that desmin stains positive, and actin HHF-35 stains negative in this case. Assuming that 0% positive rate means that 100% stain negative, the probability that pleomorphic liposarcoma shows these immunohistochemistry results at hand is:
- 17% x 100% = 17%
The gross likelihood of pleomorphic liposarcoma therefore becomes:
- 30% x 17% = 5.1%
In the same way, the corresponding gross likelihood of pleomorphic rhabdomyosarcoma is calculated as:
- 70% x 95% x (100% - 71%) = 19%
As a result in this case, immunohistochemistry resulted in pleomorphic rhabdomyosarcoma going from about twice as likely compared to pleomorphic liposarcoma to about 4 times as likely. Although results like these do not make major differences individually, detailed calculations of results from a large panel of immunohistochemistry stains can have a major impact when taken together, even if each stain has relatively low sensitivity and specificity.[note 3]
Consultation
Reporting
Following are general notes on reporting in pathology.
- Save your digital work frequently, and also before you leave a computer, even if you think you'll be back shortly. If you have many small specimens to write up in the same report, you may want to save every 2 to 3 specimens. It doesn't matter how much time and effort you spend on something if you're just going to let it disappear in the next glitch.
- Double-check your report, especially if you copy-pasted and adapted a previous report rather than using a template with blank fields or making your report from scratch.
Components
Selection and trimming
From the stage of selection and trimming, a histopathology report should preferably include:
- Case:
- Patient identification and/or sample number
- Type of tissue sample as described on container
Microscopic evaluation
- Specimen chronology, often A, B, C, etc., at least where there are multiple specimens from the same case. With multiple specimens, preferably write out the chronology for all of them first, so that you don't miss reporting any of them later.
- Specimen type and/or surgery type, such as "appendix, appendectomy", for clarification. This is not necessary at all departments. For the procedure, use the same term as the operating report whenever possible.
- Microscopic description. This is not always necessary, but should be included if the diagnosis is uncertain. One systematic approach is to describe findings from largest to smallest ones. For example, a description of a tumor can start with the demarcation of the tumor, followed by texture, cell shapes, nucleus shapes and chromatin appearance.
- Diagnosis or most probable diagnoses.
- If the diagnosis does not clearly account for all conditions that were requested, suspected or asked to be ruled out by the referring clinician (such as stated on the requisition form), you need to classify the specimen as "positive for" versus "negative for" for each such condition, or give a reason for why an evaluation thereof could not be made.
- In case of malignancy or suspected malignancy:
- Depth or most distant invasion of malignant findings.[16] Depending on location, it may need to exclude important pathways, such as vascular, neural and/or through capsules or other layers.
- Whether the resection is radical or not.
Depth
Factors supporting a relatively more comprehensive report, particularly in the inclusion of negated findings:
- Lack of explanation from existing evidence. For example, an inflamed appendix that fits the medical history does not need detailed mention of harmless incidental findings.
- Prospective review: If your report is likely to undergo double-reading by another pathologist before sign-out, it should either be more detailed, because the doctor who will do the double-reading then gets an idea of your thought process, including what you have looked for versus what may still need to be evaluated. If you know who will do the prospective review for a report of yours, you may alternatively convey your thought process by other means such as directly talking to that person.
- Highly suspected locations, such as given from the referral.
- Difficulty in obtaining the specimen, such as a CT-guided biopsy versus a skin shave.
- Defensive precautions, which appears to be more common among doctors in the Unites States compared to for example Europe.[17][18]
Multiple instances of the same type of pathology (such as lung nodules) can often simply be reported as such, at least with a particular mention of the largest or the most severe example thereof.
Uncertainty
|
| probably likely |
| suggesting |
| suspicious for |
| possibly |
| (benign condition) cannot be excluded |
| not likely |
| (malignant condition) cannot be excluded |
|
When something looks very much like a specific entity but you are not sure, preferably use "-like" (or when feasible, "-oid" such as squamoid for squamous-like cells).
When the clinical picture strongly favors a certain condition, and the pathology favors it as well, findings are generally described as "consistent with". Sometimes, "bordering on" can be described when the picture almost fits specified criteria of a specific diagnosis.
It is alright to consider the diagnosis of a pathology report to be a combination of the clinical picture and what can be seen on the specimen. For example, if the microscopy picture is uncertain, you may to a certain degree tend towards the diagnosis that best fits the clinical picture. However, mention differential diagnoses if they are still significantly possible, and would confer a different treatment or another substantially different consequence.
For both findings and diagnoses, is preferable to write "negative for..." rather than "no..." to emphasize the possibility of false negative findings.
Synoptic reports
For cancers, generally include a synoptic report, such as per College of American Pathologists (CAP) protocols at cap.org/protocols-and-guidelines. However, synoptic reports are generally not needed for tumor metastases.
Sizes
Whenever possible, give numerical quantities of sizes, rather than descriptions that are subjective (such as "small" or "large") or variable (such as "apple-sized").
Tailoring
The information contained in the reporting sections in this resource assume that the clinician has requested the exam for the topic at hand, but should still be tailored to any particular questions or requests by the clinician. Any relevant findings beyond the issues or questions raised by the clinician should also be mentioned.
As there are generally local practices about how to write reports, it is recommended to have either a personal or practice-specific repository of report templates that you can copy-paste for various cases. When doing so, however, use marks or empty spaces for relevant findings that are frequently changed in the template. Such marks should be conspicuous, so that the report clearly looks unfinished if you haven't tailored it to the case at hand (such as "...measuring _____."). Thereby, you avoid omissions or even wrongly entered information from templates.
The most important findings are preferably moved to near the top of the report if feasible.
Example of report format:
A. Organ, side/site, procedure:
|
Wording
If a certain grammatical rule has a risk of making the report less clear to the reader, ignore that rule in that situation.
Restrict acronyms/abbreviations to those who are certainly well known among all doctors, such as "cm".[note 4]
Generally describe what can be seen rather than processes (such as preferring "an abundance of" rather than "proliferation of").
If using a dictation device, avoid "no", and instead use "negative for" (and "positive for" in opposite cases), since there's a risk of "no" not being transcribed and thereby creating the opposite meaning.
Avoid "evidence of" unless you feel comfortable with the legal implications of its meaning in the report.
Whenever there is text needing formatting (text size, font type and/or UPPER vs lower case), it is generally most efficient to do it all at once after all the text is written.
Skin excisions
In skin cancers, use "peripheral" or "radial" margins (whereas "lateral" margin should be reserved for the margin opposite to the medial margin).[19]
Gastrointestinal pathology
Appendix
Gross processing
- Measure the length of the appendix.[20]
- ((Note its shape.[20] ))
- Inspect the serosa (color, congestion, adhesions, hemorrhage, exudate etc)
- ((Describe and measure the mesoappendix.))
- Cut off about 1.5 cm from the tip and split it in half long the lumen. Divide the remainder into about 3-5 mm thick transverse slices.[20]
- ((Note the wall thickness[20]))
- Look mainly for:[20]
- Luminal pus or obstruction, including stones.
- Look for any yellow firm areas at the tip, which may be carcinoids. Carcinoids may hide behind obstructions in the tip. If found grossly, submit entire appendix, and ink the surgical margin and submit separately en face[note 5]
- Wall defects
- Exterior coatings. Note if it contains "stones" or fruit kernels.
- Any tumor. If found:
- Measure the greatest dimension of the tumor
- Look for foci of carcinoma or lymph nodes in the mesoappendiceal fat, which may be lymphatic or perineural invasion
Tissue selection
- Submit:[20]
- At least one half of the tip
- At least one slice from visually inflamed areas.
- ((A transverse slice closest to the base, that is, the surgical cut.))
- ((At least one transverse slice from an intermediate part.))
Particular findings indicating additional sampling include:[20]
- Wall discoloration
- External green-gray-yellow coating
- Suspected wall defects
Submit the entire appendix if:
- There is excessive amounts of mucus, (including a representative section of any free mucus if the eppendix is perforated).
- The surgical report mentions perforation but none is found grossly.
- The surgical report mentions appendicitis but no significant inflammation is found grossly.
Gross report
Example:
((Labeled - appendix. The specimen is received in formalin and consists of a resected)) appendix measuring __ cm in length and __ cm in maximum diameter. The serosa is tan-red {{and
The attached mesoappendix measures __ cm and appears {{
On cut sections, the lumen {{is dilated and contains {{
No perforation is identified. ((Representative sections are submitted for microscopic examination in __ cassette(s).)) |
See also: General notes on gross processing
Microscopic evaluation
Always look for inflammation and malignancy.
Inflammation
- Main article: Appendicitis
Neutrophilic infiltrates of the wall of the appendix in the correct clinical context confers a diagnosis of appendicitis.
Malignancy
- Look for cancerous cells (also for specimens with clinical appendicitis).
- Look in particular for carcinoid tumors of the distal tip.
- In the presence of mucus, look for any mucinous neoplasm.
Appendiceal carcinoid. The arrow points out a cluster of neuroendocrine cells. There are also inflammatory cells consistent with acute appendicitis.[21]
Low-grade appendiceal mucinous neoplasm: Minimal cytological atypia of the epithelial cells.[22]
Report
- Description of objective findings.
- Presence or absence of malignancy.
Example, using image at right:
|
Mucosa with ulceration. ((No atypia in residual mucosa.)) Inflammatory cells in the stroma and all muscle layers, as well as on the serosal surface and adherent adipose tissue. No evidence of malignancy. ((Optionally: No perforation)) |
For cancers, generally include a synoptic report, such as per College of American Pathologists (CAP) protocols at cap.org/protocols-and-guidelines.
See also: General notes on reporting
Notes
- ↑ Further information on what is memorization-worthy or not: Learning pathology
- ↑ 2.0 2.1 The author has no financial or other conflict of interest in the mentioning of ImmunoQuery.
- ↑ More detailed explanations about likelihood calculations on differential diagnoses in general can be read at:
-Häggström, Mikael (2014). "An epidemiology-based and a likelihood ratio-based method of differential diagnosis ". WikiJournal of Medicine (Wikiversity Journal of Medicine) 1 (1). doi:. ISSN 2002-4436. - ↑ Acronyms/abbreviations increase reading speed only if the reader is familiar with the abbreviated terms:
- ↑ En face means that the section is tangential to the region of interest (such as a lesion) of a specimen. Further information: Gross_processing#Cutting
Main page
References
- ↑ Okayama K, Ishii Y, Fujii M, Oda M, Okodo M (2022). "Causation of cornflake artifacts: Possible association of poor dehydration with drying before mounting in Papanicolaou stain. ". Diagn Cytopathol. doi:. PMID 35712848. Archived from the original. .
- ↑ Gary Gill. RE: Cornflaking. Histosearch. Retrieved on 2022-09-01.
- ↑ 3.0 3.1 . Cancer staging. National Cancer Institute. Retrieved on 4 January 2013.
- ↑ 4.0 4.1 . General oncology. Amboss. Retrieved on 2020-01-29.
- ↑ List of included entries and references is found on main image page in Commons: Wikimedia Commons: Metastasis sites for common cancers.svg
- ↑ Lymph nodes, liver, lungs, bones, or skin are the main sites of cancer of unknown primary origin (CUP):
. Cancer of Unknown Primary Origin. Memorial Sloan Kettering Cancer Center. Retrieved on 20222-10-14. - ↑ Choi JH, Ro JY (2020). "Epithelioid Cutaneous Mesenchymal Neoplasms: A Practical Diagnostic Approach. ". Diagnostics (Basel) 10 (4). doi:. PMID 32316685. PMC: 7236000. Archived from the original. .
- ↑ 8.0 8.1 8.2 Lin F, Liu H (2014). "Immunohistochemistry in undifferentiated neoplasm/tumor of uncertain origin. ". Arch Pathol Lab Med 138 (12): 1583-610. doi:. PMID 25427040. Archived from the original. .
- ↑ Partly inspired by:
Beauchamp K, Moran B, O'Brien T, Brennan D, Crown J, Sheahan K (2023). "Carcinoma of unknown primary (CUP): an update for histopathologists. ". Cancer Metastasis Rev. doi:. PMID 37394540. Archived from the original. . - ↑ 10.0 10.1 Selves J, Long-Mira E, Mathieu MC, Rochaix P, Ilié M (2018). "Immunohistochemistry for Diagnosis of Metastatic Carcinomas of Unknown Primary Site. ". Cancers (Basel) 10 (4). doi:. PMID 29621151. PMC: 5923363. Archived from the original. .
- ↑ Elliot Weisenberg, M.D.. Esophagus - Carcinoma - Adenocarcinoma. Pathology Outlines. Last author update: 1 June 2013. Last staff update: 31 October 2022
- ↑ 12.0 12.1 12.2 Collado Martín R, García Palomo A, de la Cruz Merino L, Borrega García P, Barón Duarte FJ, Spanish Society for Medical Oncology (2014). "Clinical guideline SEOM: cancer of unknown primary site. ". Clin Transl Oncol 16 (12): 1091-7. doi:. PMID 25392080. PMC: 4239766. Archived from the original. .
- ↑ Partly inspired by:
Beauchamp K, Moran B, O'Brien T, Brennan D, Crown J, Sheahan K (2023). "Carcinoma of unknown primary (CUP): an update for histopathologists. ". Cancer Metastasis Rev. doi:. PMID 37394540. Archived from the original. . - ↑ Elliot Weisenberg, M.D.. Esophagus - Carcinoma - Adenocarcinoma. Pathology Outlines. Last author update: 1 June 2013. Last staff update: 31 October 2022
- ↑ Martin SE, Temm CJ, Goheen MP, Ulbright TM, Hattab EM (2011). "Cytoplasmic p63 immunohistochemistry is a useful marker for muscle differentiation: an immunohistochemical and immunoelectron microscopic study. ". Mod Pathol 24 (10): 1320-6. doi:. PMID 21623385. Archived from the original. .
- ↑ 16.0 16.1 16.2 16.3 . An Example of a Melanoma Pathology Report. Melanoma Foundation. Retrieved on 2019-09-24.
- ↑ Studdert D. M.; Mello M. M.; Sage W. M.; DesRoches C. M.; Peugh J.; Zapert K.; Brennan T. A. (2005). "Defensive medicine among high-risk specialist physicians in a volatile malpractice environment ". JAMA 293 (21): 2609–2617. doi:. PMID 15928282.
- ↑ Steurer J.; Held U.; Schmidt M.; Gigerenzer G.; Tag B.; Bachmann L. M. (2009). "Legal concerns trigger PSA testing ". Journal of Evaluation in Clinical Practice 15 (2): 390–392. doi:. PMID 19335502.
- ↑ David Slater, Paul Barrett. Standards and datasets for reporting cancers - Dataset for histopathological reporting of primary cutaneous basal cell carcinoma. The Royal College of Pathologists. February 2019
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 20.6 Monica Dahlgren, Janne Malina, Anna Måsbäck, Otto Ljungberg (1997-02-13). Lilla utskärningen.
- ↑ Elkbuli, Adel; Sanchez, Carol; McKenney, Mark; Boneva, Dessy (2019). "Incidental neuro-endocrine tumor of the appendix: Case report and literature review ". Annals of Medicine and Surgery 43: 44–47. doi:. ISSN 20490801.
- ↑ Hajjar, Roy; Dubé, Pierre; Mitchell, Andrew; Sidéris, Lucas (2019). "Combined Mucinous and Neuroendocrine Tumours of the Appendix Managed with Surgical Cytoreduction and Oxaliplatin-based Hyperthermic Intraperitoneal Chemotherapy ". Cureus. doi:. ISSN 2168-8184.
Image sources
Appendicitis
Gross processing
Standard sections if the appendix appears inflamed and there are no signs of malignancy. Describe abnormal signs including:
Further information: Appendix
Microscopic evaluation
- Evaluate depth of the inflammation.
- Look for any perforation of the wall.
- Look for cancerous cells (which may have caused the appendicitis). Further information: Appendix
- (Attempt to specify the type of appendicitis as either of the following:)
Types
| Pattern | Gross pathology | Light microscopy | Image | Clinical significance |
|---|---|---|---|---|
| Acute intraluminal inflammation | None visible |
|

|
Probably none |
| Acute mucosal inflammation | None visible |
|
May be secondary to enteritis. | |
| Suppurative acute appendicitis | May be inapparent.
|
|

|
Can be presumed to be primary cause of symptoms |
| Gangrenous/necrotizing appendicitis |
|
|

|
Will perforate if untreated |
| Periappendicitis | May be inapparent.
|
|

|
If isolated, probably secondary to other disease |
| Eosinophilic appendicitis | None visible |
|
Possibly parasitic, or eosinophilic enteritis. | |
| Chronic appendicitis[2] |
|
|
Should preferably correlate with long-term or recurrent symptoms. |
Further workup
(In acute suppurative appendicitis, still look for any periappendicitis. Also look by the lumen for parasites.)
Microscopy report
Should include, if detected:
- Acute or chronic appendicitis
- Depth of inflammation
- Any abscess and\or perforation
- Necrosis and\or ulceration, at least if transmural
(Classification into one or several types as per table above.)
- Example
| (Appendix, resection (or appendectomy):) Acute (suppurative) appendicitis (and periappendicitis) with transmural necrosis and perforation. |
Gallbladder
Gross processing
Cholecystectomy grossing
- Describe the serosa (smooth and intact versus disrupted, adhesions, inflammation, tumor implants, necrosis, porcelain).
- (Inspect the adventitia (the roughened juxtahepatic surface), where disruptions are generally iatrogenic and optional to report.)
- Cut off the cystic duct margin and submit
- Look for any cystic duct lymph node, describe and submit if present
- Open the gallbladder longitudinally on the serosal surface. Do not open along the adventitia.[note 1]
- (Estimate the amount of bile.)
- {{Estimate the number and describe gallstones.}}
- Describe the mucosa (such as velvety, granular, trabeculated, and/or with cholesterol stippling)
Cholesterol stippling (cholesterolosis): yellow streaks of cholesterol deposition, a frequent incidental finding.[3]
- Look for any gallbladder polyps or tumors. Tumors will usually be hard.
- Open the spiral neck, and look for lesions and gallstones therein
- Cut through the wall, and look for any tumors or Rokitansky-Aschoff sinuses
- Look for gallstones in the container
- Gross report
((Labeled - gallbladder. The specimen is received in formalin and consists of a resected)) {{
gallbladder measuring __ cm in length and __ cm in maximum diameter. The serosa is
Upon opening, the lumen contains ((__ [[volume in cc/cm3]]))
bile {{and
gallstones measuring up to __ cm in greatest dimension.}} The mucosa is
The spiral neck is patent {{/ obstructed by one additional similar gallstone measuring __ cm}}. The wall measures up to __ cm in thickness. {{ Rokitansky-Aschoff sinuses are present within the fundus. There is a __ (color) cystic duct lymph node present measuring __ cm in greatest dimension. }} ((Representative sections are submitted for microscopic examination in __ cassette(s). )) |
Rifts on the adventitial side that are consistent with surgical trauma need mentioning only in tumor cases.
Carcinoma
Pathology trainees that find an unsuspected tumor should generally notify a senior before continuing.
- State whether the gallbladder is intact when you received it.
- Ink the surgical margin (adventitial surface).
- Ink the cystic duct margin (lightly) and put in a separate cassette. Notify the lab to have it submitted en face[note 2]
- Look for any cystic duct lymph nodes. If found, bisect and submit.
- Measure the tumor in greatest dimension and thickness and state where in the gallbladder it is located (fundus, body, etc and whether it is on the peritoneal or hepatic side).
- Measure the margin to the cystic duct resection
- State all other abnormalities including stones, Rokitansky-Aschoff sinuses etc.
Take sections from:
- Cystic duct margin, en face
- Cystic duct lymph node if present
- Sections of tumor, full thickness
- Sections of unaffected gallbladder
Autopsy grossing
The gallbladder and biliary tract may be cut open from either end:
- Starting from the gallbladder: Cut the gallbladder open and from there dissect the cystic duct and common bile duct through the ampulla of Vater.
- Starting from the duodenum: Identify the ampulla of Vater, possibly by bile flow when squeezing the gallbladder. Dissect the common bile duct, cystic duct and thereafter the gallbladder. If the cystic duct is difficult to find, transverse cuts may be performed at its presumed location.
- In the gallbladder, inspect the contents and the appearance of the wall. Look mainly for signs of carcinoma. Optionally, estimate the volume of bile therein.
- In the biliary tract, look mainly for stones and stenosis.
Further information: Autopsy
Fixation
Generally 10% neutral buffered formalin.
See also: General notes on fixation
Microscopic evaluation
Look at least at the epithelial lining, for atypia and inflammation (such as edema and inflammatory cells, Further information: cholecystitis ).
Acute cholecystitis.
Other findings
Report
Example:
(Gallbladder, resection:)
|
In cholecystitis:
| (Gallbladder, cholecystectomy:) <<Acute and/or chronic>> cholecystitis. {{Cholelithiasis.}} |
For cancers, generally include a synoptic report, such as per College of American Pathologists (CAP) protocols at cap.org/protocols-and-guidelines.
Cholecystitis
Inflammation of the gallbladder:
Gross processing
As per basic Gallbladder.
Microscopic evaluation
Look for signs of acute or chronic cholecystitis. If you find either, keep looking for the other as well, in case it is both acute and chronic.
Findings in acute cholecystisis
Main initial features are edema and hemorrhage.[4]
- Initially often also congestion and fibrin deposition in and around the muscular layer.[5]
- Later often necrosis of the mucosa and and deeper layers, with neutrophils.[5]
- Variable reactive epithelial changes, which may resemble dysplasia.[5]
There may be fresh thrombi within small veins.[5]
Neutrophils are only sometimes seen and are not necessary for the diagnosis.[6]
Findings in chronic cholecystitis
Typical features are:[7]
- Smooth muscle hypertrophy in the muscularis
- Mild inflammatory infiltrates
- Rokitansky-Aschoff sinuses
Other findings favoring the diagnosis are:[7]
- Granulomas (from ruptured Rokitansky-Aschoff sinuses) strongly favor the diagnosis.
- Hyalinized collagen
- Dystrophic calcification
- Lymphoid aggregates
- Atrophic and/or ulcerated mucosa
- Metaplastic changes, such as gastric or intestinal mucosa
For a general gallbladder screening, see gallbladder.
Lymph nodes
Look for reactive lymphadenopathy or metastasis. Further information: Lymph node
Microscopy report
Report:
- Relevant findings from the evaluation.
- Any cholelithiasis as per the gross report.
- Template
On this resource, the following formatting is used for comprehensiveness:
- Minimal depth
- (Moderate depth)
- ((Comprehensive))
- Other legend
<< Decision needed between alternatives separated by / signs >>
{{Common findings / In case of findings}}
[[Comments]]
Link to another page
| (Gallbladder, cholecystectomy:) <<Acute and/or chronic>> cholecystitis. {{Cholelithiasis.}} |
Gallbladder polyp
Fixation
Generally 10% neutral buffered formalin.
Gross processing
As per gallbladder, with addition to submitting the entire polyp unless very large.
Microscopic evaluation
Look for signs of the most common polyp types:
Gallbladder polyp types by relative incidence.[8]
Endoscopic gastrointestinal biopsies
Gross processing
- Count the number of fragments. They can be classified as “multiple” at over 5 or 6 specimens. Possibly add “Mixed with luminal material”.
- Preferably stain with eosin if any fragment is smaller than about 0.3 cm
- Biopsies that are thicker than about 3-4 mm generally need to be bisected.
Microscopic examination
(Read the endoscopy report before evaluating (except for polyp biopsies, where it can be presumed that the purpose is to look for any malignancy).)
Example normal reports
Further information in main articles of each location.
| (Middle third esophagus, biopsy:) Squamous mucosa without significant histopathologic changes. (Negative for eosinophilic esophagitis.) |
| (GE junction, biopsy:) Squamous and gastric mucosa without significant histopathologic changes. (Negative for intestinalized (Barrett's) mucosa.) |
| (Stomach, biopsy:) Gastric mucosa without significant histopathologic changes. ((Negative for Helicobacter pylori organisms on H&E slide.)) |
| (Small bowel, biopsy:) Small intestinal mucosa without significant histopathologic changes. ((Negative for celiac disease.)) |
- Terminal ileum
| (Small bowel, biopsy:) Small intestinal mucosa without significant histopathologic changes. ((Negative for ileitis.)) |
| (Colon, biopsy:) Colonic mucosa without significant histopathologic changes. ((Negative for colitis.)) |
Esophagus
Microscopic evaluation
On esophageal biopsies, look at least for esophagitis:
Esophagitis
Look for signs of (reflux) esophagitis, mainly:[9]
- Inflammatory cells, especially when intra-epithelial. Neutrophils confer a diagnosis of acute inflammation, while plasma cells, eosinophils and excess T cells confer a diagnosis of chronic inflammation. In eosinophil-predominant inflammation, also evaluate as suspected eosinophilic esophagitis.
- Basal cell hyperplasia exceeding 15 - 20% of the epithelial thickness.
- Stromal papillae reaching upper third of the epithelium.
- Loss of orientation of superficial epithelial cells.
- Ballooned squamous cells
Microscopy report
Example normal report for an esophagus biopsy:
| (Middle third esophagus, biopsy:) Squamous mucosa without significant histopathologic changes. ((Negative for eosinophilic esophagitis.)) |
Example report with signs of reflux:
| (Mid esophagus, biopsy:) Squamous mucosa with reactive changes consistent with reflux. ((Negative for eosinophilic esophagitis.)) |
Eosinophilic esophagitis
Microscopic diagnosis
It is characterized by a prominent eosinophilic infiltrate in the esophagus. Count in at least one high power field of an intraepithelial area where there may be the highest concentration of eosinophils, and count them. Only count eosinophils where you see the nucleus. If you know your high power field (HPF) is about 0.25 mm2, diagnose it if you see over 15 intra-epithelial eosinophils per HPF.[10] [note 3] If your HPF is significantly different, or if you are not sure, diagnose it at over 60 eosinophils/mm2. Further information: Evaluation
(Also look for other "minor criteria" associated with eosinophilic esophagitis: extreme basal zone hyperplasia with papillary hyperplasia, eosinophils concentrated in the surface epithelium as opposed to the base, eosinophilic microabscesses, eosinophil degranulation, surface desquamation, lamina propria fibrosis.[11])
Further workup
For cases of eosinophilic esophagitis, also look for any fungal organisms.
Report
- If positive:
- Presence of eosinophilic esophagitis, or findings consistent thereof
- Number of eosinophils per HPF
- (Presence or absence of fungal organisms)
Example in a positive case:
| Squamous mucosa with increased intraepithelial eosinophils (up to 80 per high-power field)(, consistent with eosinophilic esophagitis. Negative for fungal organisms (H&E stained slide). See comment.) (Comment: Increased numbers of intraepithelial eosinophils in the squamous mucosa can be found in both reflux esophagitis and eosinophilic esophagitis. Although clinical correlation is recommended, some secondary histologic features favor eosinophilic esophagitis.) |
In borderline cases, such as an incidental finding of eosinophils at around 15/HPF:
| (Esophagus, biopsy:) Squamous mucosa with increased intraepithelial eosinophils of up to 15/HPF. See comment. ... Comment: There is no evidence of eosinophilic microabscesses. Although this may represent reflux esophagitis, a diagnosis of eosinophilic esophagitis is considered in the correct clinical setting. |
Gastroesophageal junction
Comprehensiveness
On this resource, the following formatting is used for comprehensiveness:
- Minimal depth
- (Moderate depth)
- ((Comprehensive))
Microscopic examination
The main findings to look for are:
- Intestinalized mucosa (Barrett's esophagus)
- (Reflux) esophagitis.
- Gastritis. Further information: Stomach
- Esophageal adenocarcinoma
Barrett's esophagus
The main diagnostic sign of Barrett's esophagus is the presence of goblet cells. A true goblet cell should have rounded shape, clear to bluish cytoplasmic mucin, and be randomly scattered.[12] The mucin usually indents the nucleus.[12]
Histopathology of Barrett's esophagus, showing intestinalized epithelium with goblet cells, as opposed to normal stratified squamous epithelium of the esophagus, and pseudostratified columnar epithelium of the fundus of the stomach. H&E stain.
In incomplete intestinal metaplasia, there are both foveolar cells and goblet cells, the latter (indicated by arrows) usually having a slightly bluish color compared to the apical cytoplasm of foveolar cells. An occasional but specific sign of goblet cells is crescent shaped nuclei (seen in middle one).
Further information: Barrett's esophagus
Esophagitis
Look for signs of (reflux) esophagitis, mainly:[9]
- Inflammatory cells, especially when intra-epithelial. Neutrophils confer a diagnosis of acute inflammation, while plasma cells, eosinophils and excess T cells confer a diagnosis of chronic inflammation. In eosinophil-predominant inflammation, also evaluate as suspected eosinophilic esophagitis.
- Basal cell hyperplasia exceeding 15 - 20% of the epithelial thickness.
- Stromal papillae reaching upper third of the epithelium.
- Loss of orientation of superficial epithelial cells.
- Ballooned squamous cells
Report
(Document the type of mucosa:
- If both gastric and squamous mucosa is present in the same fragment, report as "Gastroesophageal junctional mucosa with..."
- If not, report the presence of squamous and/or gastric mucosa.)
Examples:
| (GE junction, biopsy:) Squamous mucosa without significant histopathologic changes. (Negative for gastric mucosa or intestinalized (Barrett's) mucosa.) |
| (GE junction, biopsy:) Gastroesophageal junctional mucosa with chronic inflammation and reactive changes(, non-specific. Negative for intestinalized (Barrett's) mucosa.) |
In case of multiple signs of reflux esophagitis:
| (GE junction, biopsy:) Gastroesophageal junctional mucosa with changes consistent with reflux esophagitis. (Negative for intestinalized (Barrett's) mucosa.) |
Barrett's esophagus
Microscopic examination
Generally, the main finding to look for in biopsies from the esophagus is intestinalized mucosa (Barret's esophagus), which is defined as the presence of columnar epithelium with goblet cells.[13] A true goblet cell should have rounded shape, clear to bluish cytoplasmic mucin, and be randomly scattered.[14] The mucin usually indents the nucleus.[14]
Further workup of intestinalized mucosa
If intestinalized mucosa (Barret's esophagus) is present, look for dysplasia:
Adenocarcinoma Further information: Esophageal adenocarcinoma
Also perform a screening for esophagitis. Further information: Gastroesophageal junction
Microscopy report
Incomplete Barret's esophagus does not need specific mention:
| (GE junction, biopsy:) Gastroesophageal mucosa with chronic inflammation and intestinal metaplasia, consistent with Barrett's esophagus. Negative for dysplasia. |
Esophageal adenocarcinoma
Mainly present in endoscopic biopsy of the gastroesophageal junction or distal esophagus.
Microscopic evaluation
In biopsies, classify as either low, moderately or high differentiated.
Well differentiated: >95% gland composition Moderately differentiated 50%-95% gland composition, Poor differentiation is <50% gland composition.[15]
Reporting
| Esophagus, biopsy: Invasive adenocarcinoma, moderately differentiated. |
Stomach
Microscopic evaluation
Generally screen for:
Gastric adenomas and adenocarcinomas. Further information: Stomach tumor
Reactive gastropathy, which is a triad of:[18]
- Foveolar hyperplasia (black arrow), generally seen as a tortuosity in the "neck" region of the gastric glands.
- Scant acute and chronic inflammatory cells (white arrow).
- Smooth muscle hyperplasia (black oval)((Look for Helicobacter pylori even without signs of gastritis.))[note 4]
Microscopy report
Example in case of normal findings:
| (Gastric, biopsy:) Gastric (oxyntic/antral) mucosa without significant histopathologic changes. (Negative for Helicobacter ((pylori{{Comprehensive-end} organisms on H&E slide.) |
Gastric polyp
Microscopic evaluation

Gastric hyperplastic polyp: Elongated, tortuous, and cystic foveolae separated by edematous and inflamed stroma.[20]
A fundic gland polyp displays cystically dilated glands lined by chief cells and parietal cells, and possibly also mucinous foveolar cells.[21]
A fundic gland polyp, low magnification.[image 1]
A gastric tubular adenoma, high magnification, showing normal gastric gland at left compared to adenoma at right, with crowded, enlarged and hyperchromatic nuclei.[image 1]
A gastric tubular adenoma, low magnification, showing hyperchromatic glands.[image 1]
Fundic gland polyp
Microscopic evaluation
A fundic gland polyp displays cystically dilated glands lined by chief cells and parietal cells, and possibly also mucinous foveolar cells.[21]
A fundic gland polyp, low magnification.[image 1]
Differential diagnosis
Gastric hyperplastic polyp: Elongated, tortuous, and cystic foveolae separated by edematous and inflamed stroma.[22]
Workup
Look for dysplasia (center), compared to normal mucosa (at right). [image 1]
Dysplasia in fundic gland polyp is mainly seen as nuclear enlargement, hyperchromasia, pseudostratification, and loss of cytoplasmic mucin.[23] However, you don't need to spend much effort in the decision, since these patients have an excellent prognosis whether there is dysplasia or not.
Reporting
Generally, keep it short:
| (Stomach, excision:) Fundic gland polyp |
Gastritis
Author:
Mikael Häggström [note 5]
Inflammation of the stomach. If biopsy is at the esophagus, evaluate as gastroesophageal junction.
Comprehensiveness
On this resource, the following formatting is used for comprehensiveness:
- Minimal depth
- (Moderate depth)
- ((Comprehensive))
Microscopy evaluation
Look for chronic or acute gastritis. If either is present, still look for the other.
Chronic gastritis
- Chronic gastritis[24]
- Presence of plasma cells, lymphocytes, and occasionally lymphoid follicles. Scattered single plasma cells and lymphocytes is normal, and the threshold is subjective, but one definition of chronic gastritis is when seeing chronic inflammation at 4x magnification (as increased dots separating glands)[25] Eosinophils and neutrophils may be present.
- Reduced mucin in the cytoplasm
- Enlargement of nuclei and nucleoi
- Subnuclear vacuolation in antral glands or pits (which is PAS negative)
- Intestinal metaplasia: with partial replacement of the mucosa of the antrum and body with metaplastic goblet cells of intestinal morphology, absorptive cells and Paneth cells.
Updated Sydney System for visual classification of gastritis.[26]
Extensive atrophy of oxyntic glands in fundus/corpus causes pseudo-pyloric metaplasia.[26]
When there is at least (mild or) moderate gastritis, especially if relatively superficial, also evaluate as a stomach biopsy for Helicobacter pylori.
Acute gastritis
- Mild acute gastritis:[16]
- Modest edema of lamina propria
- Vascular congestion
- Scattered neutrophils
- Mucosal hemorrhage
- Intact epithelium
Early acute superficial gastritis: Marked neutrophilic infiltrates appear in the mucous neck region and lamina with a pit micoabscess.[26]
- Moderate to severe acute gastritis:[16]
- Loss of superficial epithelium above the muscularis mucosa
- Hemorrhage
- Variable infiltrate with neutrophils
- Fibrinopurulent luminal exudate
- Nearby epithelium may show regenerative changes
Microscopy report
- Mild and/or chronic gastritis and severity
- (If present, state if positive or negative for Helicobacter pylori organisms.)
Chronic gastritis without neutrophils is preferably also termed "non-active".
Example:
(Stomach, biopsy:)
|
Notes
- ↑ If a tumor is found, then the adventitial surface is likely the closest surgical margin, and should therefore be spared during initial opening in order to allow for optimal sections later.
- ↑ En face means that the section is tangential to the region of interest (such as a lesion) of a specimen. Further information: Gross_processing#Cutting
- ↑ It has also been described as ≥15 intraepithelial eosinophils in ≥2 hpfs or ≥25 in any single hpf.
- Parfitt, Jeremy R; Gregor, James C; Suskin, Neville G; Jawa, Hani A; Driman, David K (2005). "Eosinophilic esophagitis in adults: distinguishing features from gastroesophageal reflux disease: a study of 41 patients ". Modern Pathology 19 (1): 90–96. doi:. ISSN 0893-3952. - ↑ H. pylori is very unlikely without gastritis or reactive changes.
- ↑ For a full list of contributors, see article history. Creators of images are attributed at the image description pages, seen by clicking on the images. See Patholines:Authorship for details.
- ↑ The combination of atrophy and gastritis (especially when deeper than submucosal) helps the clinician to potentially make a diagnosis of atrophic gastritis.
Main page
References
- ↑ Unless otherwise specified in rows, reference is:
- Carr, Norman J. (2000). "The pathology of acute appendicitis ". Annals of Diagnostic Pathology 4 (1): 46–58. doi:. ISSN 10929134. - ↑ Sierakowski, Kyra; Pattichis, Andrew; Russell, Patrick; Wattchow, David (2016). "Unusual presentation of a familiar pathology: chronic appendicitis ". BMJ Case Reports: bcr2015212485. doi:. ISSN 1757-790X.
- ↑ Talwar, OP; K.C., Geetika (2014). "Histomorphological changes in gall bladder diseases and its association with helicobacter infection
". Journal of Pathology of Nepal 4 (8): 617–622. doi:. ISSN 2091-0908.
- "Figures - available via license: CC BY 4.0" - ↑ Mills, Stacey E; Carter, Darryl; Greenson, Joel K; Reuter, Victor E; Stoler, Mark H (2009). Sternberg's Diagnostic Surgical Pathology (5th ed.). Lippincott Williams & Wilkins. ISBN 978-0781779425.
- ↑ 5.0 5.1 5.2 5.3 Hanni Gulwani. Gallbladder & extrahepatic bile ducts - Cholecystitis. Pathology Outlines. Topic Completed: 1 September 2012. Minor changes: 5 September 2019
- ↑ . Diseases of the Gallbladder. Abdominal Key (2019-09-29).
- ↑ 7.0 7.1 Hanni Gulwani. Gallbladder - Cholecystitis - Chronic cholecystitis. Topic Completed: 1 September 2012. Revised: 9 January 2020
- ↑ Pickering, Oliver; Pucher, Philip H.; Toale, Conor; Hand, Fiona; Anand, Easan; Cassidy, Sheena; McEntee, Gerry; Toh, Simon K.C. (2020). "Prevalence and Sonographic Detection of Gallbladder Polyps in a Western European Population ". Journal of Surgical Research 250: 226–231. doi:. ISSN 00224804.
- ↑ 9.0 9.1 Elliot Weisenberg. Esophagus - Esophagitis - Reflux esophagitis / gastroesophageal reflux disease. Pathology Outlines. Topic Completed: 1 October 2012. Minor changes: 8 July 2020
- ↑ Dellon, Evan S. (2012). "Eosinophilic esophagitis ". Current Opinion in Gastroenterology 28 (4): 382–388. doi:. ISSN 0267-1379.
- ↑ Ryan C. Braunberger, M.D., Joshua A. Hanson, M.D.. Eosinophilic esophagitis. Pathology Outlines. Last staff update: 20 December 2022
- ↑ 12.0 12.1 Dipti M. Karamchandani. Esophagus - Premalignant - Barrett esophagus. Topic Completed: 19 March 2020, Minor changes: 29 June 2020
- ↑ . Barrett Esophagus. Stanford University School of Medicine. Retrieved on 2020-09-01.
- ↑ 14.0 14.1 Dipti M. Karamchandani. Esophagus - Premalignant - Barrett esophagus. Topic Completed: 19 March 2020, Minor changes: 29 June 2020
- ↑ https://www.sciencedirect.com/science/article/abs/pii/S2590030723000454
- ↑ 16.0 16.1 16.2 Elliot Weisenberg. Stomach - Gastritis - Acute gastritis. pathologyOutlines. Topic Completed: 1 August 2012. Minor changes: 31 August 2020
- ↑ Elliot Weisenberg. Stomach - Gastritis - Chronic gastritis. PathologyOutlines. Topic Completed: 1 August 2012. Minor changes: 31 August 2020
- ↑ Genta, RM. (Nov 2005). "Differential diagnosis of reactive gastropathy. ". Semin Diagn Pathol 22 (4): 273-83. PMID 16939055.
- ↑ García-Alonso, Francisco Javier; Martín-Mateos, Rosa María; González-Martín, Juan Ángel; Foruny, José Ramón; Vázquez-Sequeiros, Enrique; Boixeda de Miquel, Daniel (2011). "Gastric polyps: analysis of endoscopic and histological features in our center ". Revista Española de Enfermedades Digestivas 103 (8): 416–420. doi:. ISSN 1130-0108.
- ↑ Groisman, Gabriel M.; Depsames, Roman; Ovadia, Baruch; Meir, Alona (2014). "Metastatic Carcinoma Occurring in a Gastric Hyperplastic Polyp Mimicking Primary Gastric Cancer: The First Reported Case
". Case Reports in Pathology 2014: 1–5. doi:. ISSN 2090-6781.
- Attribution 3.0 Unported (CC BY 3.0) license - ↑ 21.0 21.1 Naziheh Assarzadegan, M.D., Raul S. Gonzalez, M.D.. Stomach Polyps - Fundic gland polyp. PathologyOutlines. Topic Completed: 1 November 2017. Minor changes: 11 December 2019
- ↑ Groisman, Gabriel M.; Depsames, Roman; Ovadia, Baruch; Meir, Alona (2014). "Metastatic Carcinoma Occurring in a Gastric Hyperplastic Polyp Mimicking Primary Gastric Cancer: The First Reported Case
". Case Reports in Pathology 2014: 1–5. doi:. ISSN 2090-6781.
- Attribution 3.0 Unported (CC BY 3.0) license - ↑ Levy MD, Bhattacharya B (2015). "Sporadic Fundic Gland Polyps With Low-Grade Dysplasia: A Large Case Series Evaluating Pathologic and Immunohistochemical Findings and Clinical Behavior. ". Am J Clin Pathol 144 (4): 592-600. doi:. PMID 26386080. Archived from the original. .
- ↑ Elliot Weisenberg. Stomach - Gastritis - Chronic gastritis. PathologyOutlines. Topic Completed: 1 August 2012. Minor changes: 31 August 2020
- ↑ Lysandra Voltaggio, Johns Hopkins Department of Pathology (2018-10-31). Gastritis: A Pattern Based Approach. Arizona Society of Pathologists.
- ↑ 26.0 26.1 26.2 26.3 Carrasco G, Corvalan AH (2013). "Helicobacter pylori-Induced Chronic Gastritis and Assessing Risks for Gastric Cancer.
". Gastroenterol Res Pract 2013: 393015. doi:. PMID 23983680. PMC: 3745848. Archived from the original. .
Figures - available via license: Creative Commons Attribution 3.0 Unported
Image sources
- ↑ 1.0 1.1 1.2 1.3 1.4 Image(s) by: Mikael Häggström, M.D. Public Domain
- Author info
- Reusing images
Stomach biopsy for Helicobacter pylori
Microscopic evaluation
Look for:
- Inflammation, typically a chronic form of gastritis with germinal centers (follicular gastritis), and plasma cells in lamina propria.[1][note 1] There should be at least 3 plasma cells facing each other to make a diagnosis of chronic gastritis.
- When there is at least (mild or) moderate gastritis, especially if relatively superficial, go to high magnification and look for Helicobacter pylori-like bacteria in the lumen. They are curved, spirochete-like bacteria, generally in the superficial mucus layer and along microvilli of epithelial cells.[1]
Perform immunohistochemistry for H. pylori in cases of moderate to severe chronic gastritis, or even just one neutrophil within the epithelium, where H. pylori is not seen on H&E stains.[2]
Example report
| Stomach, biopsy: Chronic active gastritis. |
Chronic gastritis without H. pylori-like organisms can be described as non-specific:
| Mild chronic gastritis, which is non-specific. Negative for H. pylori-like organisms on H&E stain. |
Stomach tumor
Microscopic evaluation

In addition to a general screening (Further information: Stomach ), look for atypical or expanded areas, and attempt top classify by at least the most common forms.
Reporting
For cancers, generally include a synoptic report, such as per College of American Pathologists (CAP) protocols at cap.org/protocols-and-guidelines.
Gastric sleeve
Gross processing
- Measure length and maximum diameter, as well as the length of the staple line.
- Inspect the serosal surface.
- Open longitudinally
- Inspect the mucosa.
- Measure the maximum wall thickness.
Tissue selection
2 representative sections, in addition to any visible lesions.
Gross report
Example:
| ((A. Labeled - ___. The specimen is received in formalin and consists of)) a segment of pink-tan stomach measuring __ cm in length and __ cm in maximum diameter. A staple line (surgical margin) is present which measures __ cm in length. The serosal surface is pink-tan and {{focally ragged with scattered transmural defects}}. A minimal amount of soft, yellow perigastric fat is present. Upon opening, the gastric lumen contains a small amount of bloody mucoid material. The mucosa is red-tan with normal rugae and no gross lesions. The wall measures up to ___ cm in thickness. (Representative sections are submitted for microscopic examination in __ cassettes.) |
Microscopic examination
Usual stomach screening. Further information: Stomach
Example reports:
| Stomach, partial gastrectomy: Portion of stomach without significant histopathologic findings. (Negative for helicobacter pylori organisms (H&E stain).) |
| Gastric body, laparoscopic sleeve gastrectomy: Mild chronic gastritis, non specific. (Negative for H. Pylori microorganisms on H&E stained slide.) |
Duodenum
Small intestine in celiac disease
Microscopic evaluation
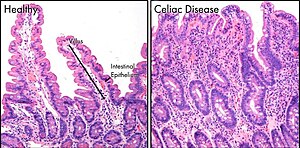
The main histologic feature of celiac disease is increased intraepithelial lymphocytes (IELs), with or without villous atrophy of the duodenal mucosa.[5] The number of intraepithelial lymphocytes are classified as follows in the duodenum:[6][7]
- < 25 IELs/100 enterocytes: Negative for intraepithelial lymphocytosis.
- 25 to 29 IELs/100 enterocytes: borderline
- > 30 IEL/100 enterocytes: Pathological "lymphocytosis"
Alternative proposed methods is the presence of over 6-12 IELs per 20 enterocytes at the tips of duodenal villi.[7] In the jejunum, the cutoff is at over 40 IELs per 100 enterocytes.[7]
Suggestive but not specific findings for enterocytes are: decreased height, intracytoplasmic vacuolation and reduction or absence of the brush border.[6]
Differential diagnoses
If findings are suggestive of celiac disease, look for at least the following differential diagnoses:
Giardia lamblia: Organisms shaped like teardropd or pears, with paired nuclei, seen in the lumen between villi.[8]
Microscopy report
Example in an unremarkable specimen:
| Small intestine, biopsy: Duodenal mucosa, negative for significant histopathologic changes. Negative for celiac disease. |
Example in suspected celiac disease:
| Small intestine, biopsy: Marked villous atrophy with increased intraepithelial lymphocytes, consistent with celiac sprue. Correlation with serologic testing is recommended. |
Colorectal polyp
Gross examination
Further information: Colon
Tissue selection and trimming

Depending on sample format:[9]
- Biopsies and polyps of <4 mm are embedded in their entirety. Samples less than 0.3 mm should be stained with eosin to avoid getting lost processing.
- Polyps 4-8 mm with short stem or without stem: Identify the excision surface and divide the polyp longitudinally through the excision surface.
- Polyps > 8 mm with a stem long enough to make it possible to take a transverse, whole slice from the stem closest to the excision surface: First, take a transverse slice through the peripheral portion of the stem, encompassing the entire circumference. Then take a 3-4 mm thick slice longitudinally through the polyp and the middle of the stem, after which the two remaining parts on either side are cut into equally thick slices, parallel to the previous slice.
- Polyps >8 mm with short stem or without stem: Identify the excision surface and cut out a 3-4 mm thick disk that extends longitudinally through the center of the excision surface. Then divide the two remaining portions into equally thick slices, parallel to the previous slice.
- Polyps that come in parts: Pick out the largest pieces, which are cut as similar as possible to above. Small fragments are sieved and embedded in a separate box.
Gross reporting
- Polyp and/or fragment sizes
- Presence or absence of stem of polyps
Example, for a gastrointestinal biopsy:
| Labeled: "Sigmoid colon biopsy". The specimen is received in formalin and consists of 4 fragments of pink-tan tissue with a vaguely recognizable mucosal surface, mixed with food-like material. The fragments measure 0.2-0.3 cm in greatest dimension. The entire specimen is submitted for microscopic examination in one cassette. |
Microscopic evaluation
Major signs
Look particularly for these, to help sorting into proper diagnosis in next section:
Main types
Consider at least the following conditions:

| Type | Risk of containing malignant cells | Histopathology | Image | |
|---|---|---|---|---|
| Hyperplastic polyp | 0% | No dysplasia.[11]
|

| |
| Tubular adenoma | 2% at 1.5cm[13] | Low to high grade dysplasia[14] | Over 75% of volume has tubular appearance.[15] | 
|
| Tubulovillous adenoma | 20% to 25%[16] | 25%-75% villous[15] | 
| |
| Villous adenoma | 15%[17] to 40%[16] | Over 75% villous[15] | 
| |
| Sessile serrated adenoma (SSA)[18] |
|

| ||
| Traditional serrated adenoma | 
| |||
| Colorectal adenocarcinoma | 100% |
|

| |
If you only see normal mucosa but the order/endoscopy says polyp, take additional levels from the paraffin block.
Other benign
If a more specific diagnosis cannot readily be made, clearly non-malignant colorectal polyps may simply be reported as such.
- Further information: Evaluation of tumors
Microscopy report
It should include:[20]
- Size of polyp (from gross examination)
- Histopathologic type
- Depth of growth and/or infiltration
- Whether the resection is radical
Optionally, it can include degree of differentiation and/or dysplasia.
Example:
| 50 mm large tubulovillous adenoma with up to high grade columnar epithelial dysplasia. No infiltration. Radical excision. |
If multiple polyps are submitted in one container, you may count the amount of each polyp type if you can, but if the amount of fragments in microscopy exceeds the amount of fragments purportedly submitted, then you can simply write "fragments of", like the following example:
| Fragments of tubular adenoma and hyperplastic polyp. |
More details are given in main articles of histopathologic types.
See also: General notes on reporting
Notes
- ↑ Plasma cells and lymphocytes are normally found in the lamina propria of the small and large intestine, but is abnormal in the stomach.
Main page
References
- ↑ 1.0 1.1 Elliot Weisenberg. Stomach - Infections - Helicobacter pylori. Pathology Outlines. Topic Completed: 1 August 2012. Minor changes: 1 September 2020
- ↑ Hartman DJ, Owens SR (2012). "Are routine ancillary stains required to diagnose Helicobacter infection in gastric biopsy specimens? An institutional quality assurance review. ". Am J Clin Pathol 137 (2): 255-60. doi:. PMID 22261451. Archived from the original. .
- ↑ Parsonnet, Julie; Friedman, Gary D.; Vandersteen, Daniel P.; Chang, Yuan; Vogelman, Joseph H.; Orentreich, Norman; Sibley, Richard K. (1991). "Helicobacter pyloriInfection and the Risk of Gastric Carcinoma ". New England Journal of Medicine 325 (16): 1127–1131. doi:. ISSN 0028-4793.
- ↑ Juwairiya Arshi, M.B.B.S., Aaron R. Huber, D.O.. Small intestine & ampulla - Malabsorption - Celiac sprue. Last author update: 4 May 2022. Last staff update: 16 September 2022
- ↑ Brown, Ian S.; Smith, Jason; Rosty, Christophe (2012). "Gastrointestinal Pathology in Celiac Disease ". American Journal of Clinical Pathology 138 (1): 42–49. doi:. ISSN 0002-9173.
- ↑ 6.0 6.1 Erdener Özer. Small intestine & ampulla, Malabsorption, Celiac sprue. Pathology Outlines. Topic Completed: 1 June 2017. Minor changes: 4 April 2020.
- ↑ 7.0 7.1 7.2 . Celiac Disease. Stanford School of Medicine. Retrieved on 2021-03-11.
- ↑ Hanni Gulwani. Small intestine & ampulla - Infectious disorders - Giardia lamblia. Pathology Outlines. Topic Completed: 1 August 2012. Minor changes: 3 March 2020
- ↑ Monica Dahlgren, Janne Malina, Anna Måsbäck, Otto Ljungberg (1997-02-13). Lilla utskärningen.
- ↑ References for pie chart are located at separate image description page.
- ↑ 11.0 11.1 11.2 Finlay A Macrae. Overview of colon polyps. UpToDate. This topic last updated: Dec 10, 2018.
- ↑ 12.0 12.1 12.2 Robert V Rouse (2010-01-31). Hyperplastic Polyp of the Colon and Rectum. Stanford University School of Medicine. Last updated 6/2/2015
- ↑ Minhhuyen Nguyen. Polyps of the Colon and Rectum. MSD Manual. Last full review/revision June 2019
- ↑ Robert V Rouse. Adenoma of the Colon and Rectum. Original posting/last update : 1/31/10, 1/19/14
- ↑ 15.0 15.1 15.2 Bosman, F. T. (2010). WHO classification of tumours of the digestive system . Lyon: International Agency for Research on Cancer. ISBN 92-832-2432-9. OCLC 688585784.
- ↑ 16.0 16.1 Amersi, Farin; Agustin, Michelle; Ko, Clifford Y (2005). "Colorectal Cancer: Epidemiology, Risk Factors, and Health Services ". Clinics in Colon and Rectal Surgery 18 (03): 133–140. doi:. ISSN 1531-0043.
- ↑ Alnoor Ramji. Villous Adenoma Follow-up. Medscape. Updated: Oct 24, 2016
- ↑ Rosty, C; Hewett, D. G.; Brown, I. S.; Leggett, B. A.; Whitehall, V. L. (2013). "Serrated polyps of the large intestine: Current understanding of diagnosis, pathogenesis, and clinical management ". Journal of Gastroenterology 48 (3): 287–302. doi:. PMID 23208018.
- ↑ 19.0 19.1 19.2 19.3 Enoch Kuo, M.D., Raul S. Gonzalez, M.D.. Colon - Polyps - Traditional serrated adenoma. Topic Completed: 1 February 2018. Minor changes: 1 October 2020
- ↑ Monica Dahlgren, Janne Malina, Anna Måsbäck, Otto Ljungberg. Stora utskärningen. KVAST (Swedish Society of Pathology). Retrieved on 2019-09-26.
Image sources
Hyperplastic polyp
Commonly presents as a colorectal polyp.
Gross evaluation
Further information: Colon
Tissue selection and trimming

Depending on sample format:[1]
- Biopsies and polyps of <4 mm are embedded in their entirety. Samples less than 0.3 mm should be stained with eosin to avoid getting lost processing.
- Polyps 4-8 mm with short stem or without stem: Identify the excision surface and divide the polyp longitudinally through the excision surface.
- Polyps > 8 mm with a stem long enough to make it possible to take a transverse, whole slice from the stem closest to the excision surface: First, take a transverse slice through the peripheral portion of the stem, encompassing the entire circumference. Then take a 3-4 mm thick slice longitudinally through the polyp and the middle of the stem, after which the two remaining parts on either side are cut into equally thick slices, parallel to the previous slice.
- Polyps >8 mm with short stem or without stem: Identify the excision surface and cut out a 3-4 mm thick disk that extends longitudinally through the center of the excision surface. Then divide the two remaining portions into equally thick slices, parallel to the previous slice.
- Polyps that come in parts: Pick out the largest pieces, which are cut as similar as possible to above. Small fragments are sieved and embedded in a separate box.
Gross reporting
- Polyp and/or fragment sizes
- Presence or absence of stem of polyps
Example, for a gastrointestinal biopsy:
| Labeled: "Sigmoid colon biopsy". The specimen is received in formalin and consists of 4 fragments of pink-tan tissue with a vaguely recognizable mucosal surface, mixed with food-like material. The fragments measure 0.2-0.3 cm in greatest dimension. The entire specimen is submitted for microscopic examination in one cassette. |
Microscopic evaluation
There are two main types of hyperplastic polyps, which have genetic differences, as well as different histologic structure, but no significant differences clinically:[2]
- A microvesicular mucin-rich type
- A goblet cell-rich type
Mucin-rich type
Characteristics:[2]
- Serrations (“saw tooth appearance”) of the luminal portion.
- Star-shaped lumina.
- Crypt elongation but they are straight, narrow and hyperchromatic at the base. All crypts reach to the muscularis mucosae.[2]
The basement membrane is frequently thickened.[2] This is the usual form of hyperplastic polyps, and they do not need to be reported as "mucin-rich".
Histologic structure in goblet cell-rich type
Elongated, fat crypts and little to no serration. Therefore, they may not be obvious without comparing to adjacent normal intestinal wall.[2]
They are filled with goblet cells, extending to surface, which commonly has a tufted appearance.[2]
Epithelial misplacement
Infrequently, the epithelium is misplacement into the submucosa. Such polyps have been termed "inverted hyperplastic polyps". They appear to be restricted to the sigmoid colon and rectum. The misplaced epithelium is mucin-depleted , similar to the basal 1/3 of the polyp. The misplacement is accompanied by the lamina propria, and is continuous with overlying polyp through a gap in the muscularis mucosae. It may require slices at multiple levels to demonstrate microscopically.[2]
In such cases adjacent hemorrhage and hemosiderin deposition is common. Collagen type IV stain will have a strong continuous staining around nests.[2]
Cellular structure
Nuclei are small, regular, round and basal in the luminal half of the crypts, most reliably evaluated near the luminal surface.[2]
There are proliferative changes at the base of crypts, where nuclei are enlarged, the nucleus/cytoplasm ratio is elevated.[2]
Differential diagnoses
The main differential diagnosis for a hyperplastic polyp is adenoma, which generally display:[2]
- Nuclear stratification
- Loss of polarity
- Dysplasia
However, the deep proliferative zones and reactive processes closely mimic changes seen in colorectal adenomas.[2]

- Sessile serrated adenoma
- A sessile serrated adenoma is suspected in case of any of the following:[2]
- Size ≥0.5 cm
- Location in right colon
If both latter findings are present, it is almost always a SSA. Other features causing a suspicion for sessile serrated adenoma are:[2]
- Dilation of crypts
- Branching of crypts
- Horizontal glands at the base
- Mature mucinous cells at the base of crypts
- Location in the proximal colon (cecum, ascending, and transverse colon),[4] whereas hyperplastic polyps are most common in the sigmoid colon and rectum. However, both may occur throughout the colon.[5]
| Hyperplastic polyp[6] | Tubular adenoma[6] |
|---|---|
| Nu dysplasia | Dysplasia |
| Proliferative epithelium restricted to base | Proliferative epithelium present at the surface |
| Gland lining cells mature at the surface | No surface maturation |
- Further information: Evaluation of tumors and Tubular colorectal adenoma
Microscopic report
Usually as follows:
| (<Sigmoid / Ascending / etc.> colon polyp, polypectomy:) Hyperplastic polyp. |
Generally don't report hyperplastic polyp elements of polyps with potential malignant progression (such as tubular and ⁄or villous adenomas), because the patient's clinical management will be based on the more concerning elements.
Tubular and ⁄or villous adenoma
Microscopic evaluation
Criteria
Dysplastic changes should involve at least the upper half of the crypts and the luminal surface.[7]
- Nuclear dysplasia is mandatory to diagnose adenoma. It involves:
- Always changes in gland architecture, such as:[7]
- Enlarged crypts
- Gland budding, or otherwise irregular glands[7]
- Mucin depletion is often seen, but is not required[7]
- Goblet cell reduction.
Differential diagnoses
| Hyperplastic polyp[6] | Tubular adenoma[6] |
|---|---|
| Nu dysplasia | Dysplasia |
| Proliferative epithelium restricted to base | Proliferative epithelium present at the surface |
| Gland lining cells mature at the surface | No surface maturation |

Colorectal carcinoma (mainly adenocarcinoma) is distinguished from an adenoma (mainly tubular and ⁄or villous adenomas) mainly by invasion through the muscularis mucosae.[9]
Also, carcinoma also commonly displays:[10]
- Varying degrees of gland formation with tall columnar cells
- Frequenty desmoplasia
- Dirty necrosis, consisting of extensive central necrosis with granular eosinophilic karyorrhectic debris. Garland of cribriform glands are frequently found in their vicinity.
Low or high grade
In low-grade lesions, the crypts should maintain a resemblance to normal colon.[11]
| Low-grade[11] | High grade[11] | |
|---|---|---|
| Crowding | Pseudo-stratification to early stratification | More substantial stratification |
| Nuclear pleomorphism and atypical mitoses | Absent or minimally present | Present |
| Loss of cell polarity | Minimal | More significant |
| Crowding, cribriform, or complex architecture | No significant | Present |
High grade lesions also typically have:
- Cribriform architecture, consisting of juxtaposed gland lumens without stroma in between, with loss of cell polarity. Rarely, they have foci of squamous differentiation (morules). This should be distinguished from cases where piles of well-differentiated mucin-producing cells appear cribriform. In such piles, nuclei show regular polarity with apical mucin, and their nuclei are not markedly enlarged.
- Increased nucleus to cytoplasm ratio.[11]
- More "open" appearing nuclei with increasingly prominent nucleoli[11]
- Back-to-back glands[11]
Tubular or villous
| Type | Histopathology | Image |
|---|---|---|
| Tubular adenoma | Over 75% of volume has tubular appearance.[12] | 
|
| Tubulovillous adenoma | 25%-75% villous[12] | 
|
| Villous adenoma | Over 75% villous[12] | 
|
Length of villi must be at least twice the depth of the normal mucosal thickness.[7]
- Further information: Evaluation of tumors
Further workup
Look for and evaluate any lymph nodes in the tissue specimen, for possible malignancy.
Dissecting pools of mucin at the base of any adenoma should be evaluated for the possibility of mucinous carcinoma.[7]
Report
- Tubular, tubulovillous or villous adenoma.
- Any high-grade component (whereas low-grade does not need to be stated in the report as it is presumed otherwise).
- ((Adenoma size))
- ((Whether it is radically resected.))
If any lymph nodes are found in the tissue sample, report the presence or absence of malignancy in any of them.
Example:
| Sigmoid colon polyp x2, polypectomies: One tubular adenoma and one tubulovillous adenoma. |
Sessile serrated adenoma
Microscopic examination
The characteristics of sessile serrated adenoma are:[13]
- Sawtooth serrations of the epithelium
- Abundant mucin, similar to hyperplastic polyps
- Basal crypt dilation, with mucous retention, and lateral spread of the crypt bases, commonly described as boot shaped or anchor shaped crypts.
Variations
On low magnification, a sessile serrated adenoma may be flat (left) or protuberant (right):[3]
Microscopic report
A brief report is sufficient:
| Cecum polyp, polypectomy: Sessile serrated adenoma. |
Differential diagnoses
Traditional serrated adenoma, having protuberant exophytic configuration, complex villous growth pattern, and cells with abundant eosinophilic cytoplasm and elongated penicillate nuclei with dispersed chromatin.[14]
Hyperplastic polyp, should not have dilation of crypts, branching of crypts or horizontal glands at the base. Size larger than 0.5[15] cm slightly indicates a sessile serrated adenoma.
Regarding location, the diagnosis of a sessile serrated adenoma is supported by a location within the proximal colon (cecum, ascending, and transverse colon), while hyperplastic polyps are most common in the sigmoid colon and rectum. However, both may occur throughout the colon.[16][17]
Traditional serrated adenoma
Microscopic evaluation
- Protuberant villi with slit-like serrations.[18]
- Pseudostratified epithelial columnar cells shapes.[18]
- Eosinophilic cytoplasm and dark, pencil-like nuclei.[18]
- Golblet cells are present.[18]
Differential diagnosis
Sessile serrated adenoma: sessile rather than protuberant exophytic configuration, and cells generally have smaller and less eosinophilic cytoplasm.[14]
Intestine with tumor and colorectal carcinoma are given later in this handbook. If you suspect a colorectal carcinoma at this time, and you are not familiar with the condition, refer the case to a senior.
Breast pathology
Breast biopsy or excision
Fresh breast specimens should be put in formalin within one hour.[note 1] Breast specimens should be immersed in formalin for 6-72 hours.[19]
Gross processing
Selection and trimming
For excision (also called lumpectomy):
- Determine total specimen size. ((Weight the specimen[20]))
- Ink margins. If sample orientations are marked, use different colors for different directions.[20]
- Palpate the specimen for masses. If felt, estimate the greatest dimension.[note 2] Compare with radiography if available.[20] Confirm the presence of any known biopsy clips, either visibly or by post-operative radiography (in order to confirm that the specimen includes the region of interest).

- Serially section the specimen.
- When performing triage of fresh lumpectomies, generally make slices 1.1 to 1.5 cm thick. When laid down and flattened, this is generally thin enough to allow for fixation over at least 6 hours, and thick enough to be further cut into 2 or 3 slices after fixation; Note the direction of up/down on each such thick slice so that the relative orientation of the thinner slices thereof is maintained. Slices with solid tumors are cut somewhat thinner since they won't flatten as much when laid down.
- When selecting tissue for submission after fixation, make 3-4 mm thick slices.[20]
- Inspect for any grossly visible lesions. If found, measure at least the greatest dimension of the lesion on the slice where it appears largest. (Also compare the greatest gross measurement (including any palpated one) with the greatest measurement at previous imaging, and note if it confers a staging discrepancy between imaging and gross dimensions, with cutoffs at 0.1 cm, 0.5 cm, 1.0 cm, 2.0 cm, and 5 cm.)
Invasive ductal carcinomas generally appear grossly as tan-white firm areas, here seen (white arrow) on a slice with inked margins. A radioactive seed (black arrow) was inserted by radiology to locate the tumor.
- Re-excisions
Tissue selection

- For lumpectomies, submit:[20]
- Entire specimen if it can fit in 3-5 slices.
- If larger:
- For relatively well demarcated tumors: 1 slice per cm of tumor (minimum of 3 slices of tumor), including both center and periphery of tumor, including closest distance to the surgical margin if possible.
- For diffuse or even invisible tumors such as often is the case for DCIS and ILC, either still submit whole, or use X-ray to guide what tissue to submit.
- Additional suspicious areas, including those indicated by radiography.
- ((Representative sections of margins in each direction.))
Breast specimens where breast cancer is possible should generally not be decalcified even when containing small calcifications, to preserve the ability to perform immunohistochemistry.
See also: General notes on gross processing
Gross report
- Size of original tissue sample, preferably in 3 dimensions.
- Description of inking
- Tumor properties, at least:
- (Description of sectioning and submissions.)
- ((Time of procurement and time of placement in formalin.[note 1]))
Example:
| (The specimen is received fresh and consists of) an irregular fragment of yellow tan fibrofatty tissue measuring 4.0 x 2.8 x 2.0 cm. (The specimen is oriented with two sutures and) the surgical margins are inked as follows: superior-blue, inferior-green, medial-red, lateral-yellow, anterior-orange, and posterior-black. {{A radioactive seed is embedded in the tissue.}} The tissue is serially sectioned {{to reveal a tan-gray, spiculated, indurated mass measuring _cm in greatest dimension. The mass is located _cm from the nearest (<<superior, inferior etc>>) margin. (The specimen is entirely submitted in sequential sections from medial to lateral in 10 cassettes. The medial and lateral margins are submitted as perpendicular sections.) The specimen was procured at _AM/PM on _/_/2020. The specimen was placed in formalin at _AM/PM on _/_/2020. |
Lymph nodes
When lymph nodes are submitted together with a biopsy or excision of a suspected or previously confirmed invasive lobular carcinoma (but not necessarily invasive carcinoma with lobular features), generally put the lymph node specimen through H&E processing at a relative rush. If you don't see any involvement on the H&E stain, order immunostain for CK AE1/AE3 in order to visualize otherwise occult lymph node involvement. The rushing of the lymph node samples allows you to have the immunostained slides by a similar time as the rest of the case.[21]
- Further information: Lymph node
Staining
Usually H&E staining.
Microscopic evaluation
If tumor is found, determine:
- Tumor size
- Malignancy
- Distance from excision margin
Malignancy
The most important is to classify a sample as either of the following:
- Benign
- Carcinoma in situ
- Invasive cancer
Most common types
| Finding | Relative incidence |
Histopathology | Image |
|---|---|---|---|
| Fibrocystic breast changes | 40% | Sclerosing adenosis (pictured), with an increase in glandular elements in addition to stromal proliferation that distorts and compresses glands.[23] | 
|
| Radial scar, seen as a fibroelastic stroma and entrapped glands radiating outward. Measure the size of these.[note 3] | 
| ||
| Usual ductal hyperplasia: Cohesive proliferation with haphazard, jumbled cell arrangement or streaming growth pattern. Cells have mild variation in cellular and nuclear size and shape.[24] | 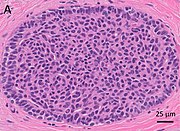
| ||
| No disease | 30% | ||
| Fibroepithelial tumor | 7% | Proliferation of both stromal and epithelial components.[note 4] The tumor group mainly includes fibroadenoma and phyllodes tumor. | 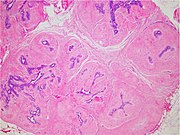
|
| Atypical ductal hyperplasia | 7%[25] | Epithelial proliferations which are not qualitatively or quantitatively abnormal enough to be classified as ductal carcinoma in situ.[25] | 
|
| Other benign mammary dysplasias and neoplasms | 5% | Including:
|

|
| Intraductal papilloma: unremarkable epithelial cells lining fibrovascular cores. | 
| ||
| Pseudoangiomatous stromal hyperplasia (PASH): Complex interanastomosing vessel-like spaces in dense collagenous, keloid-like stroma. | 
| ||
| Breast cancer (in situ or invasive) | 10% | See next section. |
Breast cancer types
| Cancer type | Histopathology | Image |
|---|---|---|
| Invasive ductal carcinoma (IDC) | Carcinomatous cells are seen below the basement membrane of lactiferous ducts. Otherwise, there are no specific histologic characteristics, essentially making it a diagnosis of exclusion.[28] | 
|
| Ductal carcinoma in situ (DCIS) | Malignant epithelial cells confined to the ductal system of the breast, without invasion through the basement membrane.[29] | 
|
| Invasive lobular carcinoma (ILC) | The "classic" pattern is round or ovoid cells with little cytoplasm in a single-file infiltrating pattern, sometimes concentrically giving a targetoid pattern. | 
|
| Lobular carcinoma in situ (LCIS) |
Cells have indistinct cell borders, pale cytoplasm, and uniform small nuclei with evenly distributed chromatin and inconspicuous nucleoli.[30] |

|
| Mucinous carcinoma | Extracellular mucin areas around tumor cells. | 
|
| Medullary carcinoma | Seemingly fused tumor cells (syncytial pattern), and a prominent lymphoid infiltrate. | 
|
| Solid papillary carcinoma | Larger tumor nests with fibrovascular cores. | 
|
- Further information: Evaluation of tumors
Previous biopsy site
(For excisions or larger, look up past biopsies, and if in the same area, look for biopsy sites in order to confirm that the previous biopsy represents the same pathologies.)
If tumor is not found
Check the imaging indication, and look for abnormalities that may explain the diagnostic findings:
- Dense stromal fibrosis for radiodense areas.
- Calcifications if found on X-ray.
If still not found, note their absence, since it indicates that the biopsy may have missed the target of interest.
Look extra close for invasive lobular carcinoma which may be subtle, as shown
Dense stromal fibrosis, in this case with entrapped fat cells and involuted entrapped ducts (arrows). In contrast, normal breast parenchyma has loose collagenous mammary stroma.[31]
Microscopy report
A normal biopsy may be reported as follows
| (Fibrofatty tissue with benign ducts and lobules.) Negative for (atypia and) malignancy. |
More detailed reports are given at the disease-related articles.
Fibrocystic breast changes
Microscopic examination
Types:
Sclerosing adenosis, with an increase in glandular elements in addition to stromal proliferation that distorts and compresses glands.[33]
Differential diagnosis
Sclerosing adenosis may look similar to invasive ductal carcinoma (IDC), but IDC will:[33]
- Not be lobular at low power
- Have marked cellular atypia
- Have no myoepithelial cells surrounding ducts.
When unsure, perform immunohistochemistry for myoepithelial markers (preferably p63 and calponin in breast lesions):
Fibroepithelial tumor
Gross examination
As per:
or mastectomy.
Microscopic evaluation
Characteristics of fibroepithelial tumor
Biplastic, having proliferation of both stromal and epithelial components,[note 5] arranged into either a pericanalicular pattern (stromal proliferation around epithelial structures), or an intracanalicular pattern (stromal proliferation compressing the epithelial structures into clefts).
Characteristics of fibroadenoma
Fibroadenomas characteristically display hypovascular stroma compared to malignant tumors.[34][35][36] Furthermore, the epithelial proliferation appears in a single terminal ductal unit and has duct-like spaces surrounded by a fibroblastic stroma. The basement membrane is intact.[37]
Characteristics of phyllodes tumor
In contrast to fibroadenomas, phyllodes tumors display a leaf-like architecture, and an increased stromal cellularity.[38] In needle biopsy specimens, phyllodes tumors can be diagnosed in a fibroepithelial tumor if there is prominent mitotic activity of ≥3 per 10 high power fields, or the finding of 3 or more of the following characteristic histologic features:[38]
- Stromal overgrowth
- Fat infiltration
- Stromal fragmentation
- Subepithelial stromal condensation
- Stromal nuclear pleomorphism
Further workup
After diagnosing a fibroepithelial tumor, classify it as benign, borderline or malignant:[39]
| Benign | Borderline | Malignant | |
|---|---|---|---|
| Stromal atypia | Mild | Moderate | Marked |
| Stromal cellularity | Mildly increased, can be focal | Moderately increased, can be focal | Markedly and diffusely increased |
| Stromal overgrowth* | Absent | Absent or very focal | Present |
| Mitotic count | < 5/10 HPF or < 2.5/mm² | 5 - 9/10 HPF or 2.5 - < 5/mm² | ≥ 10/10 HPF or ≥ 5/mm² |
| Tumor border | Well defined | Well defined or focally permeative | Diffusely permeative |
| Malignant heterologous elements | Absent | Absent | Presence directly upgrades to malignant category** |
- * Stromal overgrowth can be defined as absence of epithelial elements containing stroma only in 1 low power field.
- **Malignant categories include chondrosarcoma, liposarcoma (except well differentiated liposarcoma), osteosarcoma, rhabdomyosarcoma, angiosarcoma and leiomyosarcoma
Also, exclude another type of breast cancer, which may arise within it,[40] possibly being:
For borderline and malignant phyllodes tumors, measure the distance from the tumor to the closest surgical margin. However, such measure is not necessary for benign phyllodes tumor.[41]
Microscopic report
If, even after consultation, it is not clear whether a tumor is a fibroadenoma or phyllodes tumor, then it can be reported as a cellular fibroepithelial lesion or a fibroepithelial neoplasm. A benign lesion that resembles but does not clearly show a fibroadenoma can be called a fibroadenomatoid lesion.
A phyllodes tumor warrants a comment whether margins are positive or negative for tumor, whereas fibroadenoma does not need such comment.
Example report of fibroadenoma:
| Right breast, lumpectomy: Fibroadenoma. |
Atypical ductal hyperplasia
Gross examination
As per:
or mastectomy.
Microscopic evaluation

- A - One focus (< 2 mm) of two architecturally disarranged cross sections of tubuli showing a monotonous intraductal proliferation with secondary intraluminal architecture.
- B - One area of an ADH with associated intraluminal calcifications.
- C - Higher magnification of ADH shows low-grade nuclear atypia and monotonous cell proliferation along with secondary intraluminal architecture.
- D - Strong and uniform expression of estrogen receptors (ER). ER immunohistochemistry.
- E - Lack of basal cytokeratins (CK5/6). CK5/6 immunohistochemistry.
Atypical ductal hyperplasia shows epithelial proliferations which are not qualitatively or quantitatively abnormal enough to be classified as ductal carcinoma in situ.[25]
Differential diagnoses
There is no single definite cutoff that separates atypical ductal hyperplasia from ductal carcinoma in situ, but the following are important distinctive features of atypical ductal hyperplasia, with suggested cutoffs:[43]
- Size less than 2 mm.
- Not involving more than one duct.
- The atypical epithelial proliferation is admixed with a second population of proliferative cells without atypia.
- The proliferation completely involves the terminal ductal lobular unit(s), to a limited extent.
Also, ADH tends to have rounded lacunae between cells, in contrast to more crescent-shaped (compressed) lucunae in DCIS.
- Usual ductal hyperplasia (UDH)
In contrast to usual ductal hyperplasia (UDH), atypical ductal hyperplasia (ADH) displays:[24]
- Increased amounts of pale eosinophilic to amphophilic cytoplasm with conspicuous cell borders
- Atypical architectural patterns, including cribriform spaces, Roman arches, trabecular bars and micropapillae
If uncertain, immunohistochemistry can be performed:[24]
| Usual ductal hyperplasia (UDH) | Atypical ductal hyperplasia (ADH) | |
| High molecular weight keratins | Mosaic to occasionally diffuse | Negative |
| Estrogen receptor | Heterogenous staining | Diffusely positive |
Invasive ductal carcinoma
Gross examination
As per:
or mastectomy.
Microscopic evaluation
Malignant epithelial cells confined to the ductal system of the breast.[29] The cells are cohesive and have high grade atypia.[44]
Differential diagnoses
- Invasive ductal carcinoma
- Has invasion through the basement membrane of over 1 mm in size.[45]
DCIS with microinvasion, defined as DCIS with focus of invasive cancer measuring up to 1.0 mm in size.[46]
In uncertain cases, use immunohistochemistry stain for calponin (has the highest sensitivity) and p63 (has the highest specificity).
Immunohistochemistry for the myoepithelial marker[note 6] calponin in ductal carcinoma in situ, highlighting myoepithelial cells around all tumor cells, thereby ruling out invasive ductal carcinoma.
Invasive ductal carcinoma with tubular features can look like benign tubules, but calponin and p63 shows no surrounding myoepithelial cells.
There is no single definite cutoff, but the following are suggested cutoffs defining a ductal carcinoma in situ:[43]
- Size over 2 mm.
- Involving more than one duct.
- Lobular carcinoma in situ (LCIS)
Lobular carcinoma in situ (LCIS) displays discohesive cells, often a feathery clear space between cells, solid growth pattern, intracytoplasmic vacuoles, and lack of polarization around luminal spaces.[47]
LCIS typically fills smaller lobules rather than ducts, but DCIS can display lobular cancerization as shown at bottom of image.[image 1]
When unsure, perform immunohistochemistry for E-cadherin and p120. Both E-cadherin (left image below) and p120 (right) have a membranous staining pattern in ductal carcinoma in situ:
In contrast:
Grading
At least a low/intermediate/high grading (by Van Nuys criteria) as follows:[48]
- Low grade DCIS
- Nuclei 10-15 microns (2-3 times the size of a red blood cell)
- Nuclei oval, round, regular, evenly dispersed chromatin up to mildly irregular and minimally pleomorphic
- Nucleoi, if present, are small and indistinct
- Intermediate grade DCIS
- Same nuclear features as low grade

- Substantial tumor cell (comedo) necrosis is present
- High grade DCIS
- Nuclei >15 microns (over 2.5[51] times the size of a red blood cell)
- Nuclei are pleomorphic with clumped chromatin
- Nucleoli are prominent, enlarged
- Necrosis is almost universal and lumenal
(Numerical grading
Use the low/intermediate/high grade to give a numerical grading as follows:[52]
| Feature | Points | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| Nuclear grade | Low | Intermediate | High |
| Glands/papillae | >75% | 10% - 75% | <10% |
| Mitotic rate (per 10 HPF) | <1 | 1 - 2 | >2 |
| Central necrosis | <10% | 10% - 50% | >50% |
The points for each feature are added together, giving the following result:[52]
- 4 - 7 points: Grade 1
- 8 - 9 points: Grade 2
- 10 - 12 points: Grade 3
)
Extent
- In biopsies, do not state the extent of DCIS.
- In excisions, state the distance to the closest margin. (Additional extent specification is optional. If there is invasive tumor in addition to DCIS, you may give the DCIS extent in terms of the number of blocks containing DCIS. If an excision has DCIS without any invasive component, consider stating the total size in terms of the greatest distance between any DCIS foci.)
Microcalcifications

If invasive ductal carcinoma is seen, make at least a low power screening for microcalcifications (to correlate with imaging), but there's no need to look carefully (as tiny microcalcifications would unlikely correlate with imaging anyways).
Estrogen and progesterone receptors
Generally perform immunohistochemistry for estrogen and progesterone receptors and calculate the percentage of positive tumor cells.
HER2
((HER2 testing is not necessary, but can be done for prognostic profiling.[53])) See invasive ductal carcinoma for how to evaluate HER2.
Reporting

Example:
| Left breast mass, 2:00, 1 cm from nipple, ultrasound-guided vacuum assisted core needle biopsy: Ductal carcinoma in situ. Negative for invasive carcinoma. |
For cancers, generally include a synoptic report, such as per College of American Pathologists (CAP) protocols at cap.org/protocols-and-guidelines.
See also: General notes on reporting
Invasive lobular carcinoma
Invasive lobular carcinoma (ILC):
Gross examination
As per:
or mastectomy.
Microscopic evaluation
Characteristics
The "classic" pattern is round or ovoid cells with little cytoplasm in a single-file infiltrating pattern, sometimes concentrically giving a targetoid pattern.
Subtyping
The histologic patterns mainly include:[55][56][57]
Differential diagnosis
- In invasive lobular carcinoma, malignant cells have penetrated the basement membrane, in contrast to lobular carcinoma in situ.
- Invasive ductal carcinoma typically has duct-forming tumor cells rather than single files of tumor cells. In uncertain cases, stain for E-cadherin and p120:
Invasive ductal carcinoma, with occasional entrapped normal ducts (arrow).
E-cadherin is negative in invasive lobular carcinoma (shown), but has membranous staining in invasive ductal carcinoma
p120 has cytoplasmic staining in invasive lobular carcinoma (shown), but has membranous staining in invasive ductal carcinoma
Staging
Stage by the TNM system as follows in sections below.
Also, look for any angiolymphatic invasion. If present, check whether it reaches outside the tumor, and if so, how far.[58] Give greatest dimension (,or 3 dimensions, generally by adding up the estimated thicknesses of involved slices)).[58]
Primary Tumor (T)Tumor – Depends on the tumor at the primary site of origin, as follows:[59]
Regional Lymph Nodes (N)Lymph Node: The lymph node values depend on the number, size and location of breast cancer cell deposits in various regional lymph nodes, such as the armpit (axillary lymph nodes), the collar area (supraclavicular lymph nodes), and inside the chest (internal mammary lymph nodes.)[60][61] Each stage is as follows:[59]
Critical numbers of involved nodes: 1-3, 4-9, and 10 and over. Note any extranodal extension.[58]
Distant Metastases (M)
Overall stageA combination of T, N and M, as follows:[59]
|
- Further information: Evaluation of tumors
Grading
The Nottingham system[62] is recommended for breast cancer grading.[63] The Nottingham system is also called the Bloom–Richardson–Elston system (BRE)[64], or the Elston-Ellis modification[65] of the Scarff-Bloom-Richardson grading system.[66][67] It grades breast carcinomas by adding up scores for tubule formation, nuclear pleomorphism, and mitotic count, each of which is given 1 to 3 points. The scores for each of these three criteria are then added together to give an overall final score and corresponding grade as follows.
Tubule formation
By definition, tubule formation in classic invasive lobular carcinoma is scored as 3.[68]
Nuclear pleomorphism
Such as nuclei being larger, darker, or irregular/pleomorphic. Note: The cancer areas having cells with the greatest atypia should be evaluated.
- 1 point: nuclei with minimal or mild variation in size and shape
- 2 points: nuclei with moderate variation in size and shape
- 3 points: nuclei with marked variation in size and shape
Mitotic count
Mitotic figures are counted only at the periphery of the tumor, and counting should begin in the most mitotically active areas, and in at least 10 high-power fields (HPFs). If you know that the area of your high power field is about 0.2mm2, then you may score mitotic count as follows:[69]
- 1 point: ≤7 mitoses per 10 HPFs
- 2 points: 8 to 14 mitoses per 10 HPFs
- 3 points: ≥15 mitoses per 10 HPFs
If you have a significant different HPF area or you are not sure, count 10 HPFs and calculate the number of mitoses per mm2 (Further information: Evaluation#Counts per mm2 ):
- 1 point: ≤3 mitoses per mm2
- 2 points: 4 to 7 mitoses per mm2
- 3 points: ≥7 mitoses per mm2
A table of counts for various HPF sizes is available at the College of American Pathologists. [2] (Page 22)
Overall grade
The scores for each of these three criteria are added together to give a final overall score and a corresponding grade as follows:
- 3-5 Grade 1 tumor (well-differentiated). Best prognosis.
- 6-7 Grade 2 tumor (moderately differentiated). Medium prognosis.
- 8-9 Grade 3 tumor (poorly differentiated). Worst prognosis.
Microcalcifications

If invasive ductal carcinoma is seen, make at least a low power screening for microcalcifications (to correlate with imaging), but there's no need to look carefully (as tiny microcalcifications would unlikely correlate with imaging anyways).
Immunohistochemistry
Look at local protocols for what immunohistochemistry tests and other biomarker test need to be tested for each case of invasive breast cancer, or what needs to be retested for subsequent excisions at the primary site or at metastatic sites.[note 7]
Ki-67 index
Ki-67 index is mainly relevant in those with stage T1-T2, N0-N1, to determine if chemotherapy is needed (if Ki67 is >30% rather than <5%).[70]
Ki-67 index is most feasibly quantified by a hot spot method,[note 8] Hot spots are areas in which Ki-67 staining is particularly higher relative to the adjacent tumor areas.[71] Usually, the invasive edge of a tumor is a hot spot.[71] When a tumor had several hot spots, the “hottest” spot is selected.[71] Aim to count at least 500 cells in each case, but this is not always possible in cases with low tumor cell density and small tumor size.[71] Also aim to include at least three high-power (×40 objective) fields. Count a nucleus as “positive” if there is any definite brown staining in the nucleus of an invasive breast cancer cell, above the surrounding background in the cytoplasm and extracellular matrix.[72] If a comparisons must be made between core biopsies and sections from an excision, evaluation of the latter should be across the whole tumor.[70] Only nuclear staining counts. Staining intensity of a positive nucleus is not relevant.[70]
HER2
HER2 can initially be evaluated by immunohistochemistry (IHC) or fluorescence in situ hybridization (FISH). If IHC is performed first and is borderline/equivocal, then FISH is recommended.[73] If FISH is performed first and indicates that further workup is required, then IHC may be the performed as per established algorithms.[note 9]
HER2 immunohistochemistry
Look at different parts of the tumor, and evaluate the area(s) with most staining. When negative of faint staining is seen, evaluate at high magnification.
| Score[74][75] | Pattern[76] | Status[74][75] |
|---|---|---|
| 0 | Either:[76]
|
HER2 negative (not present) |
| 1+ | Incomplete membrane staining that is faint or barely perceptible and within >10% of the invasive tumor cells.[76] | |
| 2+ | Weak to moderate complete membrane staining observed in >10% of tumor cells.[76] | Borderline/Equivocal |
| 3+ | Circumferential membrane staining that is complete, intense, and in >10% of tumor cells.[76] | HER2 positive |
Micrographs showing each score:[77]
HER2 FISH
HER2 FISH usually uses chromosome enumeration probe 17 (CEP17) to count the amount of chromosomes. Hence, the HER2/CEP17 ratio reflects any amplification of HER2 as compared to the number of chromosomes.
To prepare a slide for HER2 testing, you may need to choose a paraffin-embedded and mark the resulting slide so that you or whoever interprets it knows where to look for the target tumor cells. When there are multiple blocks of the same case, choose the the one with most tumor. (If a block has undergone sectioning for immunohistochemistry (such as ER, PR and/or Ki67) make sure that you have a new H&E slide at a level next to the one to be used for FISH, so that they will correlate better.) In cases of both invasive and in situ carcinoma in the same specimen, mark all invasive carcinoma (also for crushed tissue or with other artifacts) but not the in situ carcinoma. Also mark a small area of normal tissue as an internal control. If possible, it should be a bit away from the tumor, even if only consisting of fatty tissue.
To interpret a HER2 FISH study, first perform a quality control check of the slide as per manufacturer and/or local protocol (generally including checking for proper signals from a control specimen). In cases of both invasive and in situ carcinoma in the same specimen, only score the invasive cells. The signals of 20 cells are usually counted. Also focus up and down on each nucleus to find all signals therein.
If a cytotechnologist has already performed a count, you do not have to recount, but make sure the count is reasonable regarding what you see. In any case, also look around for any obvious tumor heterogeneity in HER2 signals.
If the HER2/CEP17 ratio is borderline (1.8-2.2), count an additional 20 nuclei and recalculate a ratio for the total of 40 nuclei.
| HER2/CEP17 ratio | |||
|---|---|---|---|
| ≥2.0 | <2.0 | ||
| Average HER2 copy number per cell | ≥4.0 | HER2 positive | Additional work-up required[note 9] |
| <4.0 | Additional work-up required[note 9] | HER2 negative | |
If the initial HER2 result is negative for a needle biopsy of a primary breast cancer, a new HER2 test may be performed on the subsequent breast excision.[note 9]
Estrogen and progesterone receptors
Generally perform immunohistochemistry for estrogen and progesterone receptors and calculate the percentage of positive tumor cells.
Lobular carcinoma in situ
Fixation
Generally 10% neutral buffered formalin.
Microscopic evaluation
Lobular carcinoma in situ (LCIS) typically display monomorphic, loosely cohesive, slightly enlarged and evenly spaced cells that fill acini.[30] Cells have indistinct cell borders, pale cytoplasm, and uniform small nuclei with evenly distributed chromatin and inconspicuous nucleoli.[30]
Differential diagnosis
The main differential diagnosis is ductal carcinoma in situ (DCIS).
In DCIS, the cells are cohesive and have high grade atypia.[79]
LCIS typically fills smaller lobules rather than ducts, but DCIS can display lobular cancerization as shown at bottom of image.[image 1]
When unsure, perform immunohistochemistry for E-cadherin and p120:
In contrast, both E-cadherin (left image below) and p120 (right) have a membranous staining pattern in ductal carcinoma in situ (DCIS).
Microcalcifications

If invasive ductal carcinoma is seen, make at least a low power screening for microcalcifications (to correlate with imaging), but there's no need to look carefully (as tiny microcalcifications would unlikely correlate with imaging anyways).
Biomarkers
Testing for hormone biomarkers is not needed for LCIS (in contrast to ductal carcinoma in situ where ER/PR is generally indicated).
Microscopic report
It should contain:[80]
- Type of resection or biopsy, and location
- Results of any supplementary studies performed
- Extent
However, grading and staging is not applicable. (Margins of excision are not relevant)
Autopsy
The extremely minimal autopsy checklist:
- Check the autopsy referral
- Cut open and inspect the usually clearly visible organs, as well as the thyroid, pancreas and adrenals.
- Weigh relevant organs
- Sample tissues for microscopy and/or microbiology when needed
- Report at least relevant findings
Checklist for non-forensic autopsy
There are many variants, with the following being a suggestion:
Preparations
- Confirm that there is a consent from the next of kin to perform the autopsy, and whether it includes any restrictions (such as only examining the thorax).
- Read the autopsy referral (if separate from the consent form)( and talk with the referring person and/or family why they want the autopsy and what they want to be investigated in particular, if not already evident from the referral. Surprisingly often, the main reason for performing an autopsy is not the cause of death in itself, but for some other question by the clinician or relative, such as a cause of a dementia. This information helps you focus on the most pertinent locations and possible findings to answer the question.)
- Confirm with any supervisor what you may do independently, and at what stages the supervisor will want to inspect the body and/or organs (for example before evisceration and/or after all pertinent organs have been opened).[note 10]
- (Go through the patient's medical records, at least if necessary aspects are not included in the referral. The most important aspects are:
- Time of death
- Surgical history
- Current diseases
- Latest hospital and/or primary care notes
- Presence of any pacemaker or implantable cardioverter-defibrillator (ICD). If present, make sure it is deactivated before cutting open the chest. Generally remove it during autopsy as they may explode if the body is cremated.)
- ((History of smoking
- Medications, especially anticoagulant ones.
- Family history))
In the autopsy room, before starting examination:
- Charge and bring a camera to document relevant findings.
- Use protective wear, generally according to local practice. Tie any knots before putting on gloves for better dexterity.
- Confirm the identity of the body, generally from attached identity tag.
External inspection
- Measure the size of <<clinically significant / ( all)>> scars, marks, bruises or wounds. Estimate the depth of wounds, either by number or presumed anatomic layer.
- Note any therapeutic tubes or lines
- Inspect the lower legs for swelling (and compare their circumferences taken 10 cm above the ankle. If swelling is suspected, later press the legs while observing the femoral veins emptying into the pelvic cavity to discern if there is free flow.
- Discern the race, gender, nutritional status, and apparent versus stated age
- Inspect the external ears, eyes, nostrils and mouth.)
In situ inspection
- Note any atypical organ arrangement
- Estimate (or measure) excessive amount of fluid in body cavities.
- (Measure the thickness of subcutaneous fat at the thorax and abdomen.)
Evisceration
- Y-incision
- The standard Y-incision is from each acromion process to the xyphoid process. For larger breasted patients, the incision is done under the breast, making it easier to manage later.
- From here the incision extends down the central abdomen, to the patient’s left of the umbilicus, ending at the pubis. Care is taken not to cut too deeply into the abdomen, to avoid puncturing the peritoneal cavity and/or bowel.
- The skin is reflected back over the face and along the sides. Always cut perpendicular to bone.
- Take measurement of the chest and abdominal pannus at this time.
- Chest plate
- Using rib cutters or Stryker saw, cut laterally along the ribs, ending at the sterno-clavicular junction.
- Remove chest plate by detaching it from the diaphragm and pulling upward toward the head.
- Inspect the ribs for fractures
- Also, squeeze out bone marrow on a piece of wrapping tissue and submit.
- Perform in situ examination as per checklist.
- Bowel removal
- Pull up stomach and find the ligament of Treitz (pictured).
- At the ligament of Treitz, place two hemostats & cut through the bowel between the clamps.
- Cut the mesenteric adipose close to the bowel, without perforating it.
- When you reach the rectum, place two hemostats and cut through the bowel between the clamps as before.
- Supra-pelvic organ blocks
- Cut the diaphragm free from the body wall on both sides extending down to the spinal column.
- From here pull all the organs to the left and make an incision along the right side of the spinal column to free the organs
- Do the same along the left side.
- Tease the soft tissue of the neck to expose the carotid arteries.
- Tie off both sides leaving long stumps, which are marked with strings for embalming purposes.
- Dissect the tissue of the neck to expose the thyroid cartilage.
- Transect immediately above the thyroid cartilage, freeing the trachea and esophagus.
- Start to dissect away the tissue around and behind the thyroid cartilage & esophagus.
- Continue this dissection down, following the spinal column to the bifurcation of the abdominal aorta.
- At this point, remove the organ block from the body.
- Pelvic organs
- Bluntly dissect the urinary bladder away from the wall of the true pelvis
- Continue this blunt dissection down into the pelvis to include the vagina (if female) & rectum
- Pull directly anterior to free these structures
- You will hear a loud suction noise when done correctly
- At this point, cut straight down to remove the pelvic organs
- If male, insert the scissors into the pelvis toward the feet as far as possible & then down to include the prostate
- To remove the testis, bluntly dissect along the inguinal canal, exposing the spermatic cord
- Pull up on the cord until the testis pulls through the canal & out of the scrotum
- Transect the spermatic cord.
- Skull cap
- Separate the hair of the scalp, cut from ear to ear (just behind each ear) over the posterior crown of the head.
- Reflect the skin forward over the face & back to the neck, exposing the skull cap (use a towel for better grip).
- Using a scalpel, outline the cut on the anterior & posterior skull cap cutting away the temporalis muscles. The outline should make ~45 degree angle located at the level of the ear.
- Use the Stryker saw to cut along the outline. Be sure to cut through the skull bone but not into the brain tissue.
- Make a V-shaped notch in the anterior midline to help the skull cap stay in place following autopsy
- Pull skull cap off
- Brain
- Do an in situ examination before removing.
- Gently insert fingers over frontal lobes and pull backwards.
- Using scalpel/scissors, cut away the exposed cranial nerves.
- Cut away the tentorium, keeping the scalpel close to the bone.
- Free the cerebellum.
- Insert scalpel/scissors as deep as possible to cut the brain stem.
- Pull brain out gently.
- Place a wedge at level of sella turcica and hit it with a hammer to expose pituitary gland.
- Remove the gland gently.
- Weigh the brain.
Organ packages
Organs are generally initially separated into the following packages:
- Thorax
- Gastrointestinal, including liver, spleen and pancreas.
- Pelvic, including urinary bladder and uterus/prostate.
The kidneys and adrenals may be included in either the gastrointestinal or pelvic package.
To separate the thoracic from the abdominal package:
- Turn the packages with the dorsal side up.
- Optionally: Cut the aorta just distally to the exit of the left subclavian vein, and separate the descending thoracic aorta from the rest of the thorax.
- Optionally: Cut the esophagus at its superior attachment to the trachea, and separate it from the thorax.
- Free the basilar aspects of the lungs from the diaphragm.
- Identify the inferior vena cava (IVC) and transect it as close to the diaphragm as possible.
- Detach the pericardium from the diaphragm as close to the line of attachment as possible.
Heart
- Remove the parietal pericardium
- Separate the heart from the from lungs by cutting through the major vessels. The pulmonary artery should be cut first and the lumen inspected for any pulmonary embolism.
- Weigh the heart.
- Dissect the coronary vessels. Further information: Arteries
- On the right side of the heart, dissect in the direction of blood flow: Superior vena cava > right atrium > tricuspid valve > right ventricle. Look for thromboses or patent foramen ovale.[note 11]
- Dissect the atrial appendages, to exclude thromboses.
- Dissect the left ventricle, such as into circumferential slices from the apex to the base.[note 12] Inspect (and measure) the left ventricular wall thickness.
- (Measure the circumferences of the four valves. Cutoffs for valve dilatation:[81]
- Mitral valve: circumference greater than 9.9 cm in males and 9.1 cm in females
- Aortic valve: circumference greater than 8.5 cm in males and 7.9 cm in females
- Tricuspid valve: circumference greater than 11.8 cm in males and 11.1 cm in females
- Pulmonic valve: circumference greater than 7.5 cm in males and 7.4 cm in females)
Look for signs of myocardial infarction. Further information: Heart autopsy and Autopsy of myocardial infarction
Other thorax
- Dissect the aorta, with an anterior dissection of the aortic arch and major branches, and posterior dissection of the descending thoracic aorta. Check for thrombosis and degree of atherosclerosis. Then separate the descending thoracic aorta from the esophagus.
- Dissect the pulmonary arterial system, from the pulmonary trunk and including at least segmental arteries. To avoid cutting through the left main bronchus (passing anteriorly to the left main pulmonary artery), the initial dissection of the left main pulmonary artery may begin from an anterior perspective but keeping the cuts along the posterior wall, until the dissection can be seen and be continued from a posterior approach. Check the pulmonary arteries for blood clots.
| Pre-mortem | Post-mortem | |
|---|---|---|
 |

| |
| Texture | Dull | Shiny |
| Wall adherence | Yes | No |
| Color | Grey. Possibly zebraic appearance by lines of Zahn, with mixed red and grey/yellow | Dark-red ("currant jelly") to tan-pink ("chicken-fat") |
| Pressurized | Yes, can eject from lumen | No, needs to be pulled-out |
| Consistency | Firm and brittle | Elastic, jellylike |
- Esophagus: Separate it from the trachea and dissect it.
- Dissect the trachea and the bronchial tree, at least to segmental bronchi. Check for obstructions.
- Make some additional sections through the lung parenchyma. Squeeze at each side to detect any pus and edema.[83] Further information: Lung autopsy
- Cut each thyroid lobe in horizontal slices and inspect the parenchyma. Further information: Thyroid
- ((Look for parathyroid glands, and if found, inspect for nodules or hypertrophy. Further information: Parathyroid glands ))
- Look for enlarged lymph nodes in the hilar and paratracheal area.
Retroperitoneal
For orientation, the coeliac trunk and mesenteric arteries exit the aorta from the anterior side.
- Dissect the descending abdominal aorta. Cut external iliac artery from a dorsal approach, or after freeing ureters.[note 13]
- Inspect the renal arteries for patency. ((Dissect them until entry into kidneys.))
- Make a couple of cuts through the adrenal glands, such as transversal ones, and look mainly for tumors. Further information: Adrenals
- Cut the kidneys in the coronal planes. Further information: Kidney autopsy
- Dissect the ureters to the bladder. Look for any increased caliber or abnormal contents.
- Dissect the prostate in males, and the urinary bladder, by an anterior approach. Dissect the ureteropelvic junctions. Inspect the bladder content and the urothelial surface.
- Dissect the rectum.
- In females, bivalve the uterus by cutting along right and left lateral aspects. Measure the ovaries.
Peritoneal
- Remove the diaphragm and excess omentum

- Dissect the stomach along the greater curvature, as well as the duodenum and the esophageal entry into the stomach.
- Dissect the extrahepatic biliary tract and gallbladder.[note 14]Further information: Gallbladder
- Make consecutive liver slices, such as in the sagittal or coronal plane. Further information: Liver

Note its shape, color and consistency.
- Inspect the pancreas regarding color and consistency. ((Separate it and measure its dimensions.)) Cut it in consecutive short axis slices. Note the appearance of the parenchyma (and the size of the duct). Further information: Pancreas
- Separate the spleen and make consecutive short axis slices through it. Note its shape, color, texture and consistency.
- Separate the intestines until the rectum, and dissect them by a longitudinal section, looking for focal and diffuse lesions. Note the presence or absence of the appendix.
For the liver, pancreas and spleen, sectioning preferably goes almost but not fully through each organ, so as to keep the slices together.
Brain
- Weight the brain. Overall normal range (95% prediction interval) is 1100 to 1700 g,[86] +60g for males and -60g for females.[87]
- Inspect: Grooves indicating herniation? Hemorrhages?
- Dissect the basilar artery and circle of Willis, either before or after separation from the brain. [[If there is likely a need to demonstrate the case to an additional person later, the arteries of the skull base are preferably dissected after first separating them from the brain.]] Look mainly for thromboses.
- Separate the brainstem, cerebellum and cerebrum, which may be done by first separating the former two together from the cerebrum.
- Slice each part, looking for hemorrhages and/or infarcts.
- For the cerebrum, cut it into slices about 1 cm thick. It can be done from frontal to occipital, or by starting coronally into two halves at the level of the mammillary bodies and continuing in each direction from there.
- At least in people aged over 65-75 years of age {{or with suggestive history}}, look for signs of Alzhemier's disease (see picture).
Further information: Brain autopsy
Demonstration
Consider summoning involved clinicians for a demonstration of any findings.
Weighing
Separate and weigh these organs, either before or after cutting into them, but before tissue sampling.
|
|
Tissue sampling
Sample tissues for microscopy from wherever histopathology may aid in establishing the cause of death. Following are routine samples:
|
|
In addition, generally sample all gross lesions, except uterine fibroids, diverticula and multiple intestinal polyps. One sample is sufficient in case of multiple metastases. Lesion samples should include adjacent normal tissue.
Furthermore, generally save a piece of tissue in formalin from at least all sampled locations, in case the sample gets lost or gets processed incorrectly. Further information: Gross processing
Microbiology
- Indications
Cultures for microbiology are indicated in the following cases, if less than 24 hours have passed since the time of death:[note 15]
- Fever of unknown origin
- At request of the requesting physician
- (Drug addicts
- Patients from nursing homes)
- Sites
- Blood cultures from the right ventricle/atrium
- Any exudates or pus collections
- (Any organ suspected to have inflammation)
- {{Cerebrospinal fluid if meningitis is suspected}}
- {{Body fluids, by individual indication}}
Toxicology
- {{Toxicology is taken if highly suspected from the history. Up to 2.5 ml can be obtained from each eye, using a red top tube.}}
Reporting of non-forensic autopsy
- The order of the sections may vary.[88]
((Where findings are made, still negate additional findings in the region, such as: "There is a 18.0 cm curvilinear well-healed thin scar in the left thorax. Otherwise, there are no puncture marks or healed surgical scars on the torso."))
Data
- Autopsy No.: ________
- (Hospital No.:000046)
- Patient name: Bloggs, Joe
- (Patient No.:)
- (Ward :_______)
- Age [[or birthdate]]: _____
- (Sex: ______)
- (Race: ______)
- (Permission: From [[usually close relative of the deceased.]])
((The medical records of the <<patient/deceased>> [[Either denomination works]] were reviewed prior to the commencement of the autopsy.))
- Date (and time) of death: ______
- Date (and time) of autopsy: ______
- (Date of report: _________)
- Attending physician: _______
- (Prosector:__________)
- {{Limitation of the autopsy}} [[such as restriction to thorax only]].
Final autopsy diagnosis / Preliminary diagnoses
[[May be preliminary if histopathology samples are taken.]]
- [[First, generally the final cause of death, such as:]]
- {{Acute circulatory failure with pulmonary edema and congestion of abdominal organs.}}
- [[Other diagnoses that may have caused the death is listed in causative order:]]
- {{Acute myocardial infarction.}}
- {{Severe arteriosclerosis.}}
- [[Other autopsy diagnoses, including presumably incidental ones:]]
- {{Hepatic steatosis.}}
- [[Clinically known diagnoses may be marked as such:]]
- {{Type 2 diabetes (clinical diagnosis).}}
- (Clinical history
_________)
- (Laboratory data
_________)
External inspection
((The autopsy is performed approximately __ hours after death.)) The body is a ((well developed,)) << ((well nourished)) / {{underweight / overweight}}>> (__ year old) [[if not already given in data]]) << woman / man >>. ((The body appears as stated age.)) (Lengths is __ cm and weight is __ kg..)
| Usual signs of death. | (Rigor mortis is << well marked / broken >>. Lividity is seen on the << front / back and/or side >>.) |
(On the torso there are no puncture marks or healed surgical scars.)
((The skin is <<pale / icteric / cyanotic>>. The body hair shows a normal <<male / female>> distribution. There is no peripheral edema. The head is not deformed. It is covered by <<scanty / moderate / abundant>> amount of [[color:]] ____ hair. The irides are [[color:]] ___. The pupils are round, regular and equal, measuring _cm in diameter. The corneae and lenses are transparent. The sclerae are clear. The conjunctivae are <<pink / pale>>. The nose and external ears are unremarkable and their passages are clear. The lips and gums show no lesions. {{The lips are pale/cyanotic.}} The mouth is not remarkable {{/edentulous /contains a frothy fluid}}. The neck structures are symmetrical, and there are no unusual masses. {{There is jugular venous distention}}. The thorax has normal contour and symmetry. [[In females:]] breasts and nipples are unremarkable. (The back has lividity.) The abdomen is flat {{/protuberant}}. External abdominal palpation detects no abnormal masses or fluid waves. The extremities show no scars or deformities. The nails are normal {{/cyanotic/clubbed}}. No palpable inguinal masses.))
Internal examination
((Standard thoracic incisions are employed.))
{{Overall severe autolysis, making pathologic assessment difficult.}}
((The subcutaneous fat measures ___ in thickness over the thorax and _cm over the abdomen. The skeletal muscles are red-brown, normally firm and of normal bulk. There is no subcutaneous emphysema. The abdominal organs are in their usual anatomic positions. The diaphragmatic domes reach the <<sixth / seventh>> intercostal space, bilaterally.))
- Serous cavities
| No increased amount of fluid in the pericardial, pleural or abdominal cavities. Serous surfaces are smooth and lustrous.(No signs of inflammation. No adhesions.) | [[These are preferably located by the corresponding system of each cavity:]]
|
- Circulatory system
The heart << has normal weight / is enlarged [[ > 399 g in women and> 449 g in men]] >>, weighing ___ g. ((The epicardium is transparent. There is a moderate amount of subepicardial fat.
| Normal configuration | (No atrial or ventricular dilation. No ventricular wall thickening) / {{The left ventricle has {{concentric}} hypertrophy, with a wall thickness of ___ mm.}} | ((No atrial or ventricular dilation. The right and left auricular appendage is unremarkable. The left ventricular wall thickness is __ cm and the right is ___. The trabeculae carneae are normal ({[Finding-begin}}/ prominent /flattened}}.)) [[A comprehensive report may describe each atrium, valve and ventricle etc. in order of blood flow.]] |
(Foramen ovale is closed.) ((The ductus arteriosus is obliterated))
The coronary arteries ((arise in normal position. The coronary ostia are << patent {{partially occluded by arteriosclerotic calcification}}>>. They)) have << no / mild / moderate / severe >> {{and partially calcified}} arteriosclerosis. They are traced, ((throughout their length by transverse sections)) {{after fixation and decalcification}} <<without significant constrictions.{{ / The lumina of the left anterior descending, right coronary, and left circumflex coronary arteries are _%, _%, and _% narrowed, respectively.}} [[If the degree of stenosis on microscopy sections of coronary arteries only differ slightly from the gross description, preferably write "mild/moderate/severe atherosclerosis consistent with the gross inspection."]] (Gross measurement of coronary artery stenosis is generally more accurate than microscopic measurement, so the former generally has precedence.)
No thrombi in the cardiac atria (including auricles), chambers or coronary arteries.
| Chordae tendineae, the endocardium and heart valves are unremarkable. | (The endocardium is smooth and shiny. Chordae tendineae are unremarkable. The valves are normal in number, and are thin and fine at the openings.) | ((The endocardium is smooth, transparent and free of mural thrombi. The valve leaflets and chordae tendinae are overall delicate, pliable and free of lesion or calcification. <<Its leaflets are thin and pliable / No signs of inflammation>>.
{{The cusps are calcified at the bases / adherent to each other.}} {{The valve displays mild / moderate / severe myxomatous degeneration.}} The epicardium and subepicardium are unremarkable. The papillary muscles are normal {{ / hypertrophied}})) |
The myocardium has ((a homogeneous reddish brown color, and)) no signs of <<fresh lesion / ((areas of necrosis or hemorrhage))>> (or scar){{/ streaks of white scar tissue}}. In the aorta (and its major branches) there is {{widespread}} << no / mild / moderate / severe >> arteriosclerosis. No aneurysm. (Renal arteries ((are patent and)) have no significant stenosis.) No thrombus in (vena cava, ) the pulmonary arteries ((or the pulmonary veins)){{The <<right / left>> <<proximal and/or distal pulmonary arteries contain coiled, red-purple thromboemboli with(out) attachment to the intima.}} ((The portal system appears unremarkable. The vena cavae are also unremarkable. There is a free flow of blood from the veins of the lower extremities.))
- Respiratory system
| The larynx, trachea and bronchi are normally configured, with non-irritated lining. | ((Larynx has normal configuration. The vocal cords are smooth and symmetrical. The trachea and the larger bronchi have non-irritated lining. No ulceration. Lumina are patent.)) {{The bronchial lumina contain small amounts of frothy mucoid material.}} |
No foreign content((, dilatation or mucosal change)).
((Normal lobar structure. No tumor. No visible signs of inflammation.))
The lungs ((have the usual shape and lobar divisions, are expanded and)) have << normal / increased >> weight, of __ g on the right and ___ g on the left. (The consistency is normal) {{/ abnormally firm. There is <<minimal / moderate / extensive subpleural anthracotic pigment present.}}Cut Surfaces[note 16] are unremarkably dark red, with no definable tumors( or bleeding).
{{There are firm with patchy areas of tan-grey consolidation of location. Watery and frothy liquid is pressed from the parenchyma, indicating pulmonary edema.}}
(The rib cage is intact) / {{Multiple rib fractures, consistent with CPR.}} ((There are no masses in the mediastinum.))
- Digestive system
((The tongue has no bleeding.))
| The esophagus, stomach and intestines are ordinarily configured, without tumors or blood in the lumen. | ((The esophagus is lined by a smooth, tan-white non-irritated epithelium, and the mucosa has normal thickness. No diverticula, varices, mucosal tears or ulcerations. The ventricle is of normal size, with normal wall-thickness. There is unremarkable <<partially digested food / fluid in the lumen>>, with no suspicion of blood content. The mucosa has well-formed rugae and shows <<no signs of lesions / {{mild / moderate erosions, but no ulceration}}. The gastric serosal surface is smooth.)) ((The duodenum, jejunum and ileum and colon are ordinarily configured with non-irritated lining. Content is normal. The mucosa is intact throughout. No visible tumor. The ileocecal valve is competent.)) |
(The appendix is non-irritated and of normal size.)[note 14] {{There are scattered <<colonic / sigmoid>> diverticula without inflammation or perforation.}}
| Minimal | More comprehensive | Normal ranges |
| The liver weighs ___g. | The liver is << of normal size / {{enlarged}}, at ___g. | [[Men: 970-1860 g.[89] Women 600-1770g.[90].]] |
The liver surface is <<smooth ((and glistening)) {{/deformed by small and large nodules}}((, and is <<light tan / dark brown>> in color)). << Normal/ {{/ firm}} consistency. Cut surface[note 16] is normal / << (shows normal homogeneous brown parenchyma) / {{Yellowish color, indicating steatosis}} / {{dark nutmeg similar paths, indicating congestion}}. (No focal changes.)((The liver edge is _cm below the right costal margin.))
The gallbladder has (regular size,) thin walls ((lined by a velvety green mucosa)). It contains {{__ number of gallstones measuring up to __ mm. Otherwise}} normal bile ((of about ___ ml [[or cc]]. No tumor or gallstones.)) {{Mild / Moderate / Severe cholesterolosis.}} ((The gallbladder serosa is smooth.)) Further information: Gallbladder
The (extrahepatic) bile ducts are open(, ordinary and without gallstones in the lumen).
The pancreas has normal size (and shape, measuring __ )[[dimensions or weight]]. Cut surfaces[note 16] are normal((ly tan and lobulated, without bleeding or definable focal changes. The main pancreatic duct is patent.)).
- Lymphoid and endocrine organs
The spleen is of << normal size {{ / Enlarged [[> 230g]]}}>>, at ___ g. Normal consistency / {{ Firm consistency indicating of chronic venous stasis}}. Cut surfaces[note 16] have normal appearance / ( normal bluish-red color, with no definable focal changes). ((The splenic artery and vein are normal.))
| The thyroid and adrenal glands are normal bilaterally / (are ordinarily configured and with no definable focal changes on cut surfaces[note 16]). | [[These are preferably located by the corresponding system of each cavity:]]((
)) |
| No abnormal lymph nodes. | ( Lymph nodes in para-tracheal, para-aortic and abdominal regions are of normal size, texture and color.) ((No definable focal changes on cut surfaces[note 16].)) |
- Urogenital
The kidneys are equally sized / (of normal size, with a total weight of ___ g)((a weight of ___ g on the right side and ___ g on the right)).
| Sex | Weight, reference range[note 17] | ||
| Right kidney | Left kidney | Total | |
| Men[92] | 80–160 g (2.8–5.6 oz) | 80–175 g (2.8–6.2 oz) | 160-335g (5.6-12.8 oz) |
| Women[93] | 40–175 g (1.4–6.2 oz) | 35–190 g (1.2–6.7 oz) | 75-365g (2.6-12.9 oz) |
(No abnormal adhesions between the kidneys and surrounding fibrous capsules.)
The kidneys have smooth surfaces/ {{<<Finely / Coarsely>> granular brown surface, possibly indicating benign nephrosclerosis. There are a few cysts on the surface containing clear fluid}}. Cut surfaces have well-defined medulla, cortex, and papillae. {{The cortices and/or medullas are narrowed and congested. The papillary portions are intact.}}
The renal pelvis and ureters are unremarkable /( Renal pelvis and ureters have normal calibers, with non-irritated mucosal surfaces and open lumens).
The urinary bladder ((lies below the symphysis pubis and)) is unremarkable / ( is of normal size, with normal mucosa, and no tumor.)(( The urinary bladder contains __cc/ml of <<clear {{/ turbid}} urine. The wall is not thickened. The ureteral orifices are patent. The serosal surfaces are smooth and glistening {{and show <<soft / hard>> fibrous adhesions}})).
[[Either:]]
- [[Male]]: The prostate is of <<normal size (( measures _x _ x _ cm>> and has normal color. The consistency is <<normal elastic)){{/ firm / nodular}}. (No definable focal changes on cut surfaces[note 16]). The testes are ((descended {{, atrophic}} and)) without abnormal masses.(( The penis and scrotum are unremarkable.))
- [[Female]]:
| Uterus and adnexa are unremarkable.[note 14] | ((The uterus is normal. It measures __ x __ x __cm and weighs __ grams. Its serosal surface is <<smooth {{/ deformed by multiple nodules ranging between __ and _ cm}}>>. The cervix is unremarkable. The endometrial cavity is lined by a smooth atrophic velvety, tan membrane. The ovaries and fallopian tubes are normal.)) |
- Central nervous system
| The meninges and venous sinuses are unremarkable. | ((The skull is unremarkable. The calvarium is opened in the usual manner. The scalp and overlying fascia are not remarkable. The skull is <<normal in thickness {{/ somewhat thickened in the frontal areas}}. The cerebrospinal fluid is clear. The dura and venous sinuses are unremarkable. The leptomeninges are thin, shiny and non-irritated, with no visible bleeding or exudates. The superficial blood vessels are not congested. The sulci and gyri are <<normal {{/ flattened}}. |
(No visible thrombi. No epidural, subdural or subarachnoid hematoma.)
| The brain is symmetrical and weighs ___g. | ( The cerebral and cerebellar hemispheres are of equal size, and have a normal weight of ___g. [[Men: 1.180 to 1.620 g. Women: 1.030 to 1.400 g]][94][95] |
| No signs of herniation | (No grooves on the bases of the cerebrum or cerebellum.) |
((The cerebral ventricles are of normal size, with normal linings.))
Cut surfaces ((after fixation)) of the cerebrum, cerebellum and brainstem show (normal gray and white parenchyma, and) no ((encephalomalacia, ))(hemorrhages, tumors or other) focal abnormalities. ((The gyral pattern is preserved.))
The basal cerebral arteries << are ordinary / {{have mild / moderate / severe atherosclerosis}}>> without aneurysms or occlusions.
- Skeleton
Scalp and base of skull are ordinary / (without visible lesions or injuries).
((The vertebral column is unremarkable. The musculature is fairly well developed. There are no deformities of the musculoskeletal system. The bone marrow appears red and cellular.))
- {{Other organs
if evaluated.}}
Histopathology
No samples taken / {{Tissue samples have been taken from the << heart, lungs, liver and/or kidneys >> for supplementary microscopic and/or bacteriological examination.}}
((Clinicopathologic correlation))
[[Discussion of how findings relate to the probable clinical course.]]
Microscopy report
{{Overall <<moderate / severe>> post-mortem autolysis((, making interpretations on cellular level <<difficult / unfeasible>>)).}} [[The degree of autolysis may be specified for each site individually.]]
(After the location identifier, each list item can generally begin as "This section/These sections[note 18] << shows / reveals >>...)
- Breast: ((Section(s) show(s))) no focal changes. {{Non-proliferative fibrocystic changes and microcalcifications.}}
- Bone marrow from <<rib / __ [[other location]] >>: Section shows trilineage hematopoiesis. There is no evidence of malignancy. Further information: Bone marrow
- Lymph nodes at __ [[ location]]: ((Section(s) show(s)))__ [[number of]] benign{{, reactive lymph node(s) with anthracotic pigment deposition}}.
- Heart: Sections of ___ [[location]] show no evidence of infarction. Further information: Heart autopsy
- Coronary arteries: Sections of the three main coronary arteries reveal << mild / moderate / severe>> atherosclerosis, with approximately __%, __% and __% stenosis of the left anterior descending, left circumflex coronary artery and right coronary artery, respectively. Further information: Arteries
- Thyroid gland: ((Section(s) show(s))) normal follicles with no focal changes.
- Parathyroid: <<1, 2, 3, 4>> parathyroid gland(s) examined; ((Section(s) show(s))) no focal changes or signs of hyperplasia. Further information: Parathyroid
- Pituitary gland: ((Section(s) show(s))) adenohypophysis and neurohypophysis with no focal changes. Further information: Pituitary
- Liver: ((Section(s) show(s))) {{congested}} hepatic parenchyma with no focal changes. Further information: Liver
- Adrenal glands: ((Section(s) show(s))) right and left adrenal glands with no focal changes. (The cortices and medullae are unremarkable.)
- Spleen: ((Section(s) show(s))) normal distribution and architecture of the red and white pulp.
- Kidneys: ((Section(s) show(s))) both kidneys with {{<<mild / moderate / severe >> <<generalized / focal / nodular>> glomerular sclerosis, and <<mild / moderate / severe >> arteriosclerosis.}} There are no focal changes. Further information: Kidney autopsy
- Gastroesophageal junction: ((Section(s) show(s))) squamo-epithelial junction with no metaplasia or dysplasia. Further information: Gastroesophageal junction
- Pancreas: ((Section(s) show(s))) {{autolytic}} (pancreatic parenchyma with) no focal changes. Further information: Pancreas
- Lungs: {{Sections of all lobes of the lung}} show vascular congestion. (There is no evidence of edema, acute bronchopneumonia, malignancy or thromboemboli.) Further information: Lung autopsy
- Urinary bladder: ((Section(s) show(s))) <<normal {{/ autolytically denuded}}>> urothelium.
- Brain: ((Standard sections of the cerebral cortex, basal ganglia, hippocampus, thalamus/substantia nigra, medulla oblongata and cerebellum show)) intact cytoarchitecture. There is no evidence of hemorrhage, tumor, metastatic disease or vasculitis. Further information: Brain autopsy
Males:
- Testis: ((Section(s) show(s))) no focal changes. (There <<are no / are few / is a normal amount of>> visible mature spermatozoa.)
- Prostate: ((Section(s) show(s))) no focal changes {{/nodular prostatic hyperplasia. There is no evidence of malignancy}}.
Females:
- Uterus: ((Section(s) show(s))) no focal changes.
- Ovary: ((Section(s) show(s))) no focal changes bilaterally.
Postmortem bacterial growth is often seen in autopsy, generally as basophilic and equally sized tiny granules. The absence of inflammation indicates that it is a postmortem change (such as leukocytes being commensurate with red blood cells as in this image of bacteria within the fibrin of a postmortem blood clot).
See also: General notes on reporting
External applications
The standard reference range reference is duplicated because of technical reasons by being included in Patholines:Templates.
Heart autopsy
Autopsy cutting checklist
- Remove the parietal pericardium
- Separate the heart from the from lungs by cutting through the major vessels. The pulmonary artery should be cut first and the lumen inspected for any pulmonary embolism.
- Weigh the heart.
- Dissect the coronary vessels. More details in section below. Further information: Arteries
- On the right side of the heart, dissect in the direction of blood flow: Superior vena cava > right atrium > tricuspid valve > right ventricle. Look for thromboses or patent foramen ovale.[note 19]
- Dissect the atrial appendages, to exclude thromboses.
- Dissect the left ventricle, such as into circumferential slices from the apex to the base.[note 20] Inspect (and measure) the left ventricular wall thickness.
- (Measure the circumferences of the four valves. Cutoffs for valve dilatation:[96]
- Mitral valve: circumference greater than 9.9 cm in males and 9.1 cm in females
- Aortic valve: circumference greater than 8.5 cm in males and 7.9 cm in females
- Tricuspid valve: circumference greater than 11.8 cm in males and 11.1 cm in females
- Pulmonic valve: circumference greater than 7.5 cm in males and 7.4 cm in females)
In case of suspected infarction, see autopsy of myocardial infarction.
For left and right wall thickness, uppr limits of 1.5 cm and 0.5 cm, respectively, are generally used to distinguish wall thickening on autopsy.[97][note 21]
Gross examination of coronary arteries
To find the right and left coronary arteries, look distally to each corresponding aortic valve cusp.
Make longitudinal ((or transverse cuts at 3 mm intervals[98]) through:
- The right coronary artery.
- ((The right marginal artery))
- The left coronary and circumflex artery.
- The left anterior descending artery.
- ((The left marginal artery))
- ((The left diagonal branch))
- {{Any vessel grafts to the heart}}
Examine for atherosclerosis, stenosis and thrombi
(If the coronary arteries are calcified, sharply dissect them from the heart, without cutting too deeply into the muscle. Fix them in formalin, decalcify them and then cut them at 3 mm intervals.)
Estimate the percentage of any significant stenosis or occlusion. Further information: Arteries
Plaque at different percentages of atherosclerotic stenosis.[98]
The presence of a totally occlusive thrombotic mass confers a diagnosis of likely sudden cardiac death death even in the absence of microscopically visible necrosis.[98]
Weight
Cardiomegaly can be defined as a weight exceeding the 95th percentile of normal individuals, preferably adjusted for weight, size, age and gender.[99][note 22]
Microscopic examination
Myocardium
Look for:
- Signs of myocardial infarction
If one or more is present, see Autopsy of myocardial infarction
- (Optionally, also look for:)
Ruptured intercalated discs. Further information: Ruptured intercalated discs
- (Optionally, also perform a General microscopic screening.)
Reporting
This is en example report:
The heart << has normal weight / is enlarged [[ > 399 g in women and> 449 g in men]] >>, weighing ___ g. ((The epicardium is transparent. There is a moderate amount of subepicardial fat.
| Normal configuration | (No atrial or ventricular dilation. No ventricular wall thickening) / {{The left ventricle has {{concentric}} hypertrophy, with a wall thickness of ___ mm.}} | ((No atrial or ventricular dilation. The right and left auricular appendage is unremarkable. The left ventricular wall thickness is __ cm and the right is ___. The trabeculae carneae are normal ({[Finding-begin}}/ prominent /flattened}}.)) [[A comprehensive report may describe each atrium, valve and ventricle etc. in order of blood flow.]] |
(Foramen ovale is closed.) ((The ductus arteriosus is obliterated))
The coronary arteries ((arise in normal position. The coronary ostia are << patent {{partially occluded by arteriosclerotic calcification}}>>. They)) have << no / mild / moderate / severe >> {{and partially calcified}} arteriosclerosis. They are traced, ((throughout their length by transverse sections)) {{after fixation and decalcification}} <<without significant constrictions.{{ / The lumina of the left anterior descending, right coronary, and left circumflex coronary arteries are _%, _%, and _% narrowed, respectively.}} [[If the degree of stenosis on microscopy sections of coronary arteries only differ slightly from the gross description, preferably write "mild/moderate/severe atherosclerosis consistent with the gross inspection."]] (Gross measurement of coronary artery stenosis is generally more accurate than microscopic measurement, so the former generally has precedence.)
No thrombi in the cardiac atria (including auricles), chambers or coronary arteries.
| Chordae tendineae, the endocardium and heart valves are unremarkable. | (The endocardium is smooth and shiny. Chordae tendineae are unremarkable. The valves are normal in number, and are thin and fine at the openings.) | ((The endocardium is smooth, transparent and free of mural thrombi. The valve leaflets and chordae tendinae are overall delicate, pliable and free of lesion or calcification. <<Its leaflets are thin and pliable / No signs of inflammation>>.
{{The cusps are calcified at the bases / adherent to each other.}} {{The valve displays mild / moderate / severe myxomatous degeneration.}} The epicardium and subepicardium are unremarkable. The papillary muscles are normal {{ / hypertrophied}})) |
The myocardium has ((a homogeneous reddish brown color, and)) no signs of <<fresh lesion / ((areas of necrosis or hemorrhage))>> (or scar){{/ streaks of white scar tissue}}.
See also: General notes on reporting
Notes
- ↑ 1.0 1.1 An increased cold ischemia time (time from procurement to time in formalin) negatively effects the utility of immunohistochemistry.
- Khoury, Thaer (2018). "Delay to Formalin Fixation (Cold Ischemia Time) Effect on Breast Cancer Molecules ". American Journal of Clinical Pathology 149 (4): 275–292. doi:. ISSN 0002-9173. - ↑ A palpated greatest dimension before cutting is still superior to trying to align cut pieces together or mathematically adding the thicknesses of involved slices.
- ↑ Size is a major factor in whether to fully excise radial scars, at an approximate cutoff at around 1 cm.
- ↑ The proliferation of two histological components is called "biplasia", from Latin bis (“twice”) and -plasia (“formation”), or "biphasic proliferation"
- ↑ The proliferation of two histological components is called "biplasia", from Latin bis (“twice”) and -plasia (“formation”), or "biphasic proliferation" (although the latter may refer to proliferation that has two chronological phases).
- ↑ For myoepithelial markers, a combination of p63 and calponin is generally recommended for breast lesions. D2-40 is useful for highlighting lymphatics for invasion.
- ↑ If the previous biopsy was negative for ER and PR receptors, and the patient has undergone neoadjuvant chemotherapy before excision, then generally retest ER/PR on the excision. Retesting ER/PR on any excision with previously negative ER/PR on biopsy on a patient having received neoadjuvant therapy has no scientific support nor opposition.
- William M Sikov, MD, FACP, FNCBCJudy C Boughey, MD, FACSZahraa Al-Hilli, MD, FACS, FRCSI. General principles of neoadjuvant management of breast cancer. UpToDate. In breast cancer metastases, generally retest estrogen and progesterone receptors, and HER2 in the following circumstances:- If the status of the primary tumor is unknown or negative for ER/PR and/or HER2
- If the primary tumor is heterogeneous for ER/PR expression
- If the metastatic progression is unusual for the tumor characteristics
- If the relapse is unexpectedly early or late
- If unusual metastasis location
- If the initial test was performed more than 10 years ago
- If the testing turnaround time are relatively short (to reduce potential delays in patient management by retesting)
- ↑ Besides from a hot spot method of Ki67 counting, there is also a IKWG global average method which is more comprehensive. However, the inter-observer difference between the hot spot method and the 'IKWG global average is not statistically significant, and has not shown any significant difference in clinical outcome (theoretically, the area of highest Ki-67 proliferative index is probably most likely to correlate with malignant transformation and risk of metastasis, making the hot spot both more straightforward and clinically relevant than a global average).
- Reference and instructions for the IKWG global average method: Dowsett, M.; Nielsen, T. O.; A'Hern, R.; Bartlett, J.; Coombes, R. C.; Cuzick, J.; Ellis, M.; Henry, N. L.; et al. (2011). "Assessment of Ki67 in Breast Cancer: Recommendations from the International Ki67 in Breast Cancer Working Group ". JNCI Journal of the National Cancer Institute 103 (22): 1656–1664. doi:. ISSN 0027-8874. - ↑ 9.0 9.1 9.2 9.3 9.4 9.5 If additional work-up is required by FISH study, see source article for detailed algorithms:
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS (2018). "Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. ". J Clin Oncol 36 (20): 2105-2122. doi:. PMID 29846122. Archived from the original. . - ↑ Confirmation with a supervisor ought to be done each time until that person tells that it is not necessary.
- ↑ The right ventricle can alternatively be cut in circumferential slices along with the left ventricle.
- ↑ An alternative approach is to cut the left ventricle through a cut along the left lateral margin, followed by an anterior cut from the apex to the aortic root, freeing the anterior wall. Then cut through the plane of the myocardium of the anterior and posterior myocardial wall, as well as the septum, for any signs of infarction. (Dissect one or more papillary muscles for infarction.)
- ↑ Measures are taken to avoid cutting through the ureters.
- ↑ 14.0 14.1 14.2 An appearance like after extirpation such as cholecystectomy, hysterectomy (with bilateral salpingo-oophorectomy) and/or appendectomy, may be reported as "Appearance like after ___". An attempt should be made to confirm it from available medical records.
- ↑ After 24 hours, the likelihood of bacterial contamination and thereby false positive results is high, generally making cultures not indicated.
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 16.6 "Cut surface shows..." may alternatively be expressed as "On sectioning, the parenchyma is..."
- ↑ Renal weight range is the standard reference range, that is, defined as the interval between which 95% of values of a reference population fall into.
- ↑ This wording distinguishes single from multiple section, and emphasizes that sections are representative, and do not cover the entire volume.
- ↑ The right ventricle can alternatively be cut in circumferential slices along with the left ventricle.
- ↑ An alternative approach is to cut the left ventricle through a cut along the left lateral margin, followed by an anterior cut from the apex to the aortic root, freeing the anterior wall. Then cut through the plane of the myocardium of the anterior and posterior myocardial wall, as well as the septum, for any signs of infarction. (Dissect one or more papillary muscles for infarction.)
- ↑ The limit is different from those measured in life by medical imaging, because the left ventricular thicknesses are generally 3-5 mm thicker at autopsy than during life. In comparison, the average thickness of the left ventricle is up to 8 mm in women and 9 mm in men on MRI, using the 95% prediction interval for the short axis images at the mid-cavity level.
- Kawel, Nadine; Turkbey, Evrim B.; Carr, J. Jeffrey; Eng, John; Gomes, Antoinette S.; Hundley, W. Gregory; Johnson, Craig; Masri, Sofia C.; et al. (2012). "Normal Left Ventricular Myocardial Thickness for Middle-Aged and Older Subjects With Steady-State Free Precession Cardiac Magnetic Resonance ". Circulation: Cardiovascular Imaging 5 (4): 500–508. doi:. ISSN 1941-9651. - ↑ 22.0 22.1 External link: Chicago model for post-mortem classification of cardiomegaly, adjusted for weight, size, age and gender.
Main page
References
- ↑ Monica Dahlgren, Janne Malina, Anna Måsbäck, Otto Ljungberg (1997-02-13). Lilla utskärningen.
- ↑ 2.00 2.01 2.02 2.03 2.04 2.05 2.06 2.07 2.08 2.09 2.10 2.11 2.12 2.13 Robert V Rouse (2010-01-31). Hyperplastic Polyp of the Colon and Rectum. Stanford University School of Medicine. Last updated 6/2/2015
- ↑ 3.0 3.1 Liu, Cheng; Walker, Neal I; Leggett, Barbara A; Whitehall, Vicki LJ; Bettington, Mark L; Rosty, Christophe (2017). "Sessile serrated adenomas with dysplasia: morphological patterns and correlations with MLH1 immunohistochemistry
". Modern Pathology 30 (12): 1728–1738. doi:. ISSN 0893-3952.
- "This work is licensed under a Creative Commons Attribution 4.0 International License." - ↑ David Driman, MBChB FRCPC. Sessile serrated adenoma of the colon. MyPathologyReport. Updated July 23, 2021
- ↑ Author: Adrian C. Bateman, M.B.B.S., M.D.. Colon - Polyps - Hyperplastic polyp. Pathology Outlines. Minor changes: 23 September 2021
- ↑ 6.0 6.1 6.2 6.3 . Hyperplastic Polyp of the Colon and Rectum - Differential diagnoses. Stanford University School of Medicine. Retrieved on 2019-09-30.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 Robert V Rouse. Adenoma of the Colon and Rectum. Stanford University School of Medicine. Original posting/last update : 1/31/10, 1/19/14
- ↑ Mehran Taherian; Saran Lotfollahzadeh; Parnaz Daneshpajouhnejad; Komal Arora. Tubular Adenoma. National Center for Biotechnology Information. Last Update: May 30, 2020.
- ↑ Robert V Rouse. Colorectal Adenoma Containing Invasive Adenocarcinoma. Stanford University School of Medicine.
- ↑ Robert V Rouse. Adenocarcinoma of the Colon and Rectum. Stanford University School of Medicine. Original posting/updates: 1/31/10, 7/15/11, 11/12/11
- ↑ 11.0 11.1 11.2 11.3 11.4 11.5 David J. Myers; Komal Arora. Villous Adenoma. StatPearls, National Center for Biotechnology Information. Last Update: June 18, 2019.
-"This book is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/)" - ↑ 12.0 12.1 12.2 Bosman, F. T. (2010). WHO classification of tumours of the digestive system . Lyon: International Agency for Research on Cancer. ISBN 92-832-2432-9. OCLC 688585784.
- ↑ Enoch Kuo, M.D., Raul S. Gonzalez, M.D.. Colon - Polyps - Sessile serrated adenoma. PathologyOutlines. Topic Completed: 1 January 2018. Minor changes: 1 October 2020
- ↑ 14.0 14.1 Torlakovic, Emina Emilia; Gomez, Jose D.; Driman, David K.; Parfitt, Jeremy R.; Wang, Chang; Benerjee, Tama; Snover, Dale C. (2008). "Sessile Serrated Adenoma (SSA) vs. Traditional Serrated Adenoma (TSA) ". The American Journal of Surgical Pathology 32 (1): 21–29. doi:. ISSN 0147-5185.
- ↑ Andrea L Haws, MD, MS. Pathology of Serrated Colon Adenomas. Medscape. Updated: Apr 28, 2017
- ↑ Author: Adrian C. Bateman, M.B.B.S., M.D.. Colon - Polyps - Hyperplastic polyp. Pathology Outlines. Minor changes: 23 September 2021
- ↑ David Driman, MBChB FRCPC. Sessile serrated adenoma of the colon. MyPathologyReport. Updated July 23, 2021
- ↑ 18.0 18.1 18.2 18.3 Enoch Kuo, M.D., Raul S. Gonzalez, M.D.. Colon - Polyps - Traditional serrated adenoma. Topic Completed: 1 February 2018. Minor changes: 1 October 2020
- ↑ . Recommendations for HER2 Testing in Breast Cancer: ASCO – CAP Clinical Practice Guideline Update. College of American Pathologists (2013-10-17).
- ↑ 20.0 20.1 20.2 20.3 20.4 20.5 20.6 20.7 Monika Roychowdhury. Grossing (histologic sampling) of breast lesions. Pathologyoutlines.com. Topic Completed: 1 August 2012. Revised: 19 September 2019
- ↑ Chandler IP, Oommen R, Lawson CW (2003). "Invasive lobular carcinoma and cytokeratin immunohistochemistry: an audit. ". J Clin Pathol 56 (3): 240. doi:. PMID 12610108. PMC: 1769908. Archived from the original. .
- ↑ Mitchell, Richard Sheppard; Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson (2007). Robbins Basic Pathology (8th ed.). Philadelphia: Saunders. p. 739. ISBN 978-1-4160-2973-1.
- ↑ Jaya Ruth Asirvatham, M.B.B.S., Julie M. Jorns, M.D.. Breast - Fibrocystic changes - Sclerosing adenosis. Pathology Outlines. Topic Completed: 1 January 2015. Minor changes: 31 December 2020
- ↑ 24.0 24.1 24.2 Sofia Lérias, M.D., Melinda Lerwill, M.D.. Usual ductal hyperplasia. Pathology Outlines. Last author update: 11 February 2021. Last staff update: 25 April 2022
- ↑ 25.0 25.1 25.2 David J. Myers; Andrew L. Walls.. Atypical Breast Hyperplasia. StatPearls, National Center for Biotechnology Information. Last Update: February 15, 2019.
- ↑ Schnitt, Stuart J (2003). "The diagnosis and management of pre-invasive breast disease: Flat epithelial atypia – classification, pathologic features and clinical significance ". Breast Cancer Research 5 (5). doi:. ISSN 1465-542X.
- ↑ Logullo, Angela Flavia; Nimir, Cristiane (2019). "Columnar cell lesions of the breast: a practical review for the pathologist ". Surgical and Experimental Pathology 2 (1). doi:. ISSN 2520-8454.
- ↑ Peter Abdelmessieh. Breast Cancer Histology. Medscape. Retrieved on 2019-10-04. Updated: May 24, 2018
- ↑ 29.0 29.1 Siziopikou, Kalliopi P. (2013). "Ductal Carcinoma In Situ of the Breast: Current Concepts and Future Directions ". Archives of Pathology & Laboratory Medicine 137 (4): 462–466. doi:. ISSN 0003-9985.
- ↑ 30.0 30.1 30.2 30.3 Sucheta Srivastava. Breast - Noninvasive lobular neoplasia - LCIS classic. Topic Completed: 1 September 2017. Minor changes: 21 June 2020
- ↑ Nassar, Lara; Baassiri, Amro; Salah, Fatima; Barakat, Andrew; Najem, Elie; Boulos, Fouad; Berjawi, Ghina (2019). "Stromal Fibrosis of the Breast: A Spectrum of Benign to Malignant Imaging Appearances ". Radiology Research and Practice 2019: 1–6. doi:. ISSN 2090-1941.
- ↑ Radi MJ (1989). "Calcium oxalate crystals in breast biopsies. An overlooked form of microcalcification associated with benign breast disease. ". Arch Pathol Lab Med 113 (12): 1367-9. PMID 2589947. Archived from the original. .
- ↑ 33.0 33.1 Jaya Ruth Asirvatham, M.B.B.S., Julie M. Jorns, M.D.. Breast - Fibrocystic changes - Sclerosing adenosis. Pathology Outlines. Topic Completed: 1 January 2015. Minor changes: 31 December 2020
- ↑ Tavassoli, F.A., ed (2003). World Health Organization Classification of Tumours: Pathology & Genetics: Tumours of the breast and female genital organs . Lyon: IARC Press. ISBN 978-92-832-2412-9.
- ↑ Jaya Ruth Asirvatham, M.B.B.S., Carlos C. Diez Freire, M.D., Cansu Karakas, M.D., Belinda Lategan, M.D., Nat Pernick, M.D., Emily S. Reisenbichler, M.D., Monika Roychowdhury, M.D., Mary Ann Gimenez Sanders, M.D, Ph.D., Gary Tozbikian, M.D., Hind Warzecha, M.D. Senior Authors: Julie M. Jorns, M.D., Shahla Masood. Breast nonmalignant, Fibroepithelial neoplasms, Fibroadenoma. Retrieved on 2019-11-04. Revised: 31 October 2019
- ↑ Rosen, PP. (2009). Rosen's Breast Pathology (3rd ed.). ISBN 978-0-7817-7137-5.
- ↑ . Fibroadenoma of the breast.
- ↑ 38.0 38.1 Gary Tozbikian, M.D.. Breast - Fibroepithelial tumors - Fibroadenoma. Last author update: 19 July 2021. Last staff update: 22 July 2021
- ↑ Authors: Joshua J.X. Li, M.B.Ch.B., Gary M. Tse, M.B.B.S.. Breast - Fibroepithelial tumors - Phyllodes tumor. Pathology Outlines. Last author update: 2 December 2020. Last staff update: 11 January 2023
- ↑ Richard L Kempson MD, Robert V Rouse MD. Fibroadenoma of the Breast. Stanford University School of Medicine. May 27, 2006
- ↑ Cowan ML, Argani P, Cimino-Mathews A (2016). "Benign and low-grade fibroepithelial neoplasms of the breast have low recurrence rate after positive surgical margins. ". Mod Pathol 29 (3): 259-65. doi:. PMID 26743469. Archived from the original. .
- ↑ Rageth, Christoph J.; Rubenov, Ravit; Bronz, Cristian; Dietrich, Daniel; Tausch, Christoph; Rodewald, Ann-Katrin; Varga, Zsuzsanna (2018). "Atypical ductal hyperplasia and the risk of underestimation: tissue sampling method, multifocality, and associated calcification significantly influence the diagnostic upgrade rate based on subsequent surgical specimens ". Breast Cancer 26 (4): 452–458. doi:. ISSN 1340-6868. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/)
- ↑ 43.0 43.1 Tozbikian, Gary; Brogi, Edi; Vallejo, Christina E.; Giri, Dilip; Murray, Melissa; Catalano, Jeffrey; Olcese, Cristina; Van Zee, Kimberly J.; et al. (2016). "Atypical Ductal Hyperplasia Bordering on Ductal Carcinoma In Situ ". International Journal of Surgical Pathology 25 (2): 100–107. doi:. ISSN 1066-8969.
- ↑ Sucheta Srivastava, M.D.. Breast - Noninvasive lobular neoplasia - LCIS classic (Differential diagnosis section). Topic Completed: 1 September 2017. Minor changes: 17 May 2021
- ↑ . Protocol for the Examination of Resection Specimens from Patients with Ductal Carcinoma In Situ (DCIS) of the Breast, Version: 4.4.0.0. Protocol Posting Date: June 2021. College of American Pathologists.
- ↑ Image annotation by Mikael Häggström, MD, using source image from:
Moatasim A, Mamoon N (2022). "Primary Breast Mucinous Cystadenocarcinoma and Review of Literature. ". Cureus 14 (3): e23098. doi:. PMID 35464581. PMC: 8997314. Archived from the original. .
- "This is an open access article distributed under the terms of the Creative Commons Attribution License CC BY 4.0."
Source for microinvasion: . Protocol for the Examination of Resection Specimens from Patients with Ductal Carcinoma In Situ (DCIS) of the Breast, Version: 4.4.0.0. Protocol Posting Date: June 2021. College of American Pathologists. - ↑ Gary Tozbikian, M.D.. Breast - Ductal carcinoma in situ - DCIS. pathologyOutlines. Topic Completed: 20 May 2020. Minor changes: 6 May 2021
- ↑ . Ductal Carcinoma in Situ of the Breast. Stanford Medical School (2020-08-27).
- ↑ Image by Mikael Häggström, MD.
- Reference for most common definition of comedo necrosis by size:
- Harrison BT, Hwang ES, Partridge AH, Thompson AM, Schnitt SJ (2019). "Variability in diagnostic threshold for comedo necrosis among breast pathologists: implications for patient eligibility for active surveillance trials of ductal carcinoma in situ. ". Mod Pathol 32 (9): 1257-1262. doi:. PMID 30980039. Archived from the original. . - ↑ Image by Mikael Häggström, MD. References for features:
- . Ductal Carcinoma in Situ of the Breast. Stanford Medical School (2020-08-27).
- Hayward MK, Louise Jones J, Hall A, King L, Ironside AJ, Nelson AC (2020). "Derivation of a nuclear heterogeneity image index to grade DCIS. ". Comput Struct Biotechnol J 18: 4063-4070. doi:. PMID 33363702. PMC: 7744935. Archived from the original. . - ↑ Hayward MK, Louise Jones J, Hall A, King L, Ironside AJ, Nelson AC (2020). "Derivation of a nuclear heterogeneity image index to grade DCIS. ". Comput Struct Biotechnol J 18: 4063-4070. doi:. PMID 33363702. PMC: 7744935. Archived from the original. .
- ↑ 52.0 52.1 Allred, D. C. (2010). "Ductal Carcinoma In Situ: Terminology, Classification, and Natural History ". JNCI Monographs 2010 (41): 134–138. doi:. ISSN 1052-6773.
- ↑ Laura C Collins, MD, Christine Laronga, MD, FACS, Julia S Wong, MD. Ductal carcinoma in situ: Treatment and prognosis. UpToDate. Literature review current through: Sep 2022. | This topic last updated: Aug 19, 2022.
- ↑ Top and bottom left images by Mikael Häggström, MD. Bottom right image from:
Kulka J, Madaras L, Floris G, Lax SF (2022). "Papillary lesions of the breast. ". Virchows Arch 480 (1): 65-84. doi:. PMID 34734332. PMC: 8983543. Archived from the original. .
- "This article is licensed under a Creative Commons Attribution 4.0 International License" - ↑ "Association of Infiltrating Lobular Carcinoma With Positive Surgical Margins After Breast-Conservation Therapy ". Ann. Surg. 231 (6): 877–82. June 2000. doi:. PMID 10816631.
- ↑ "Synchronous bilateral invasive lobular breast cancer presenting as carcinomatosis in a male ". Am. J. Surg. Pathol. 33 (3): 470–4. March 2009. doi:. PMID 19092630.
- ↑ Fletcher's diagnostic histopathology of tumors. 3rd Ed. p. 931-932.
- ↑ 58.0 58.1 58.2 58.3 58.4 . Infiltrating Ductal Carcinoma of the Breast (Carcinoma of No Special Type). Stanford Medical School. Retrieved on 2019-10-02.
- ↑ 59.0 59.1 59.2 Originally copied from Fadi M. Alkabban; Troy Ferguson. Cancer, Breast. National Center for Biotechnology Information. Last Update: June 4, 2019. Creative Commons Attribution 4.0 International License
- ↑ "Internal mammary lymphadenopathy in breast carcinoma: CT appraisal of anatomic distribution ". Radiology 167 (1): 89–91. April 1988. doi:. PMID 3347753.
- ↑ "Internal mammary lymphadenopathy: imaging of a vital lymphatic pathway in breast cancer ". Radiographics 10 (5): 857–70. September 1990. doi:. PMID 2217975.
- ↑ Elston, CW; Ellis, IO (1991). "Pathologic prognostic factors in breast cancer. I. The value of histological grades in breast cancer. Experience from a large study with long-term follow-up ". Histopathology 19 (5): 403–10. doi:. PMID 1757079. "Republished ". Histopathology 41: 154–161. 2002. doi:.
- ↑ Oudai Hassan. What is the Nottingham combined histologic grade (modified Scarff-Bloom-Richardson grade) system for breast tumors?. Medscape. Updated: Mar 20, 2019
- ↑ Al-Kuraya, Khawla; Schraml, Peter (2004). "Prognostic relevance of gene amplifications and coamplifications in breast cancer ". Cancer Research 64 (23): 8534–8540.
- ↑ Elston CW, Ellis IO. Pathologic prognostic factors in breast cancer. I. The value of histological grades in breast cancer. Experience from a large study with long-term follow-up. Histopathology 1991, 19:403-410.
- ↑ Bloom, H.J.; Richardson, W.W. (1957). "Histological grading and prognosis in breast cancer; A study of 1409 cases of which 359 have been followed for 15 years ". British Journal of Cancer 11 (3): 359–77. doi:. PMID 13499785.
- ↑ Genestie, C.; Zafrani, B.; Asselain, B.; Fourquet, A.; Rozan, S.; Validire, P.; Vincent-Salomon, A.; Sastre-Garau, X. (1998). "Comparison of the prognostic value of Scarff-Bloom-Richardson and Nottingham histological grades in a series of 825 cases of breast cancer: Major importance of the mitotic count as a component of both grading systems ". Anticancer Research 18 (1B): 571–6. PMID 9568179.
- ↑ . Infiltrating Lobular Carcinoma of the Breast. Stanford Medicine. Retrieved on 2021-01-06.
- ↑ Error on call to Template:cite web: Parameters url and title must be specified. . College of American Pathologists. Protocol Posting Date: September 2022
- ↑ 70.0 70.1 70.2 Dowsett, M.; Nielsen, T. O.; A'Hern, R.; Bartlett, J.; Coombes, R. C.; Cuzick, J.; Ellis, M.; Henry, N. L.; et al. (2011). "Assessment of Ki67 in Breast Cancer: Recommendations from the International Ki67 in Breast Cancer Working Group ". JNCI Journal of the National Cancer Institute 103 (22): 1656–1664. doi:. ISSN 0027-8874.
- ↑ 71.0 71.1 71.2 71.3 Coleman, William B.; Jang, Min Hye; Kim, Hyun Jung; Chung, Yul Ri; Lee, Yangkyu; Park, So Yeon (2017). "A comparison of Ki-67 counting methods in luminal Breast Cancer: The Average Method vs. the Hot Spot Method ". PLOS ONE 12 (2): e0172031. doi:. ISSN 1932-6203.
- ↑ . Ki67-QC international working group: whole section scoring protocol (global method). International Ki67 in Breast Cancer Working Group (2018-11-29).
- ↑ . Breast Cancer HER2 Status. American Cancer Society. Last Revised: August 25, 2022
- ↑ 74.0 74.1 . IHC Tests (ImmunoHistoChemistry). Breastcancer.org. Last modified on October 23, 2015
- ↑ 75.0 75.1 "Human Epidermal Growth Factor Receptor 2 (HER2) in Cancers: Overexpression and Therapeutic Implications ". Molecular Biology International 2014: 852748. 2014. doi:. PMID 25276427.
- ↑ 76.0 76.1 76.2 76.3 76.4 2018 ASCO/CAP guidelines:
- . Figure 1. Algorithm for evaluation of human epidermal growth factor receptor 2 (HER2) protein expression by immunohistochemistry (IHC) assay of the invasive component of a breast cancer specimen.. College of American Pathologists: Homepage. Retrieved on 2022-09-12.
- Ahn S, Woo JW, Lee K, Park SY (2020). "HER2 status in breast cancer: changes in guidelines and complicating factors for interpretation. ". J Pathol Transl Med 54 (1): 34-44. doi:. PMID 31693827. PMC: 6986968. Archived from the original. . - ↑ Nitta H, Kelly BD, Padilla M, Wick N, Brunhoeber P, Bai I (2012). "A gene-protein assay for human epidermal growth factor receptor 2 (HER2): brightfield tricolor visualization of HER2 protein, the HER2 gene, and chromosome 17 centromere (CEN17) in formalin-fixed, paraffin-embedded breast cancer tissue sections.
". Diagn Pathol 7: 60. doi:. PMID 22647525. PMC: 3487810. Archived from the original. .
- "This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/2.0)" - ↑ Diagram and table by Mikael Häggström, MD. Adapted from: Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS (2018). "Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. ". J Clin Oncol 36 (20): 2105-2122. doi:. PMID 29846122. Archived from the original. .
- ↑ Sucheta Srivastava, M.D.. Breast - Noninvasive lobular neoplasia - LCIS classic (Differential diagnosis section). Topic Completed: 1 September 2017. Minor changes: 17 May 2021
- ↑ . Lobular Carcinoma in Situ of the Breast. Surgical Pathology Criteria. Retrieved on 2021-12-14.
- ↑ Kitzman, Dalane W.; Scholz, David G.; Hagen, Philip T.; Ilstrup, Duane M.; Edwards, William D. (1988). "Age-Related Changes in Normal Human Hearts During the First 10 Decades of Life. Part II (Maturity): A Quantitative Anatomic Study of 765 Specimens From Subjects 20 to 99 Years Old
". Mayo Clinic Proceedings 63 (2): 137–146. doi:. ISSN 00256196.
- Griffith, Christopher C.; Raval, Jay S.; Nichols, Larry (2012). "Intravascular Talcosis due to Intravenous Drug Use Is an Underrecognized Cause of Pulmonary Hypertension
- ↑ Michael Bonert. Autopsy. Page was last modified: 6 September 2016
- ↑ Burton, Julian L.; Rutty, Guy N. (2010). The Hospital Autopsy A Manual of Fundamental Autopsy Practice (3rd ed.). Oxford University Press. ISBN 978-0340965146.
- ↑ Çetýn S, Ýnanir NT, Eren F, Eren B, Dokgöz H (2015). "Wischnewsky Spots in Fatal Hypothermia: Case Report. ". Maedica (Bucur) 10 (3): 280-282. PMID 28261368. PMC: 5327835. Archived from the original. .
- ↑ Pellerito, John; Polak, Joseph F. (2012). Introduction to Vascular Ultrasonography (6th ed.). Elsevier Health Sciences. p. 559. ISBN 978-1-4557-3766-6.
- ↑ Govender, S; Lazarus, L; De-Gama, B. Z; Satyapal, K. S (2018). "Post-Mortem Brain Weight Reference Range for a Select South African Population ". International Journal of Morphology 36 (3): 915–920. doi:. ISSN 0717-9502.
- ↑ Kelley Hays; David S. (1998). Reader in Gender archaeology . Routlegde. ISBN 9780415173605. Retrieved on 2014-09-21.
- ↑ The report is partially inspired from: . Autopsy Report No. A97-015. State University of New York Health Science Center at Syracuse, Department of pathology (1997-05-26).
- ↑ Standard reference range: Molina, D. Kimberley; DiMaio, Vincent J.M. (2012). "Normal Organ Weights in Men ". The American Journal of Forensic Medicine and Pathology 33 (4): 368–372. doi:. ISSN 0195-7910.
- ↑ Standard reference range: Molina, D. Kimberley; DiMaio, Vincent J. M. (2015). "Normal Organ Weights in Women ". The American Journal of Forensic Medicine and Pathology 36 (3): 182–187. doi:. ISSN 0195-7910.
- ↑ Shamim, A; Monira, K; Manowara, B; Sabiha, M; Alim, A; Nurunnabi, ASM (1970). "Weight of the Human Thyroid Gland A Postmortem Study
". Bangladesh Journal of Medical Science 9 (1): 44–48. doi:. ISSN 2076-0299.
- In turn citing: Langer P. Discussion about the limit between normal thyroid and goiter: mini review. Endocrine regulations. 1999 March; 33(1): 39-45. - ↑ Standard reference range: Molina, D. Kimberley; DiMaio, Vincent J.M. (2012). "Normal Organ Weights in Men ". The American Journal of Forensic Medicine and Pathology 33 (4): 368–372. doi:. ISSN 0195-7910.
- ↑ Standard reference range: Molina, D. Kimberley; DiMaio, Vincent J. M. (2015). "Normal Organ Weights in Women ". The American Journal of Forensic Medicine and Pathology 36 (3): 182–187. doi:. ISSN 0195-7910.
- ↑ Standard reference range: Molina, D. Kimberley; DiMaio, Vincent J.M. (2012). "Normal Organ Weights in Men ". The American Journal of Forensic Medicine and Pathology 33 (4): 368–372. doi:. ISSN 0195-7910.
- ↑ Standard reference range: Molina, D. Kimberley; DiMaio, Vincent J. M. (2015). "Normal Organ Weights in Women ". The American Journal of Forensic Medicine and Pathology 36 (3): 182–187. doi:. ISSN 0195-7910.
- ↑ Kitzman, Dalane W.; Scholz, David G.; Hagen, Philip T.; Ilstrup, Duane M.; Edwards, William D. (1988). "Age-Related Changes in Normal Human Hearts During the First 10 Decades of Life. Part II (Maturity): A Quantitative Anatomic Study of 765 Specimens From Subjects 20 to 99 Years Old
". Mayo Clinic Proceedings 63 (2): 137–146. doi:. ISSN 00256196.
- Griffith, Christopher C.; Raval, Jay S.; Nichols, Larry (2012). "Intravascular Talcosis due to Intravenous Drug Use Is an Underrecognized Cause of Pulmonary Hypertension
- ↑ Sant’Anna, Mirella Pessoa; de Mello, Roberto José Vieira; Montenegro, Luciano Tavares; Araújo, Mônica Modesto (2012). "Hipertrofia cardíaca esquerda e direita em necropsias de hipertensos ". Revista da Associação Médica Brasileira 58 (1): 41–47. doi:. ISSN 01044230.
- ↑ 98.0 98.1 98.2 Michaud, Katarzyna; Basso, Cristina; d’Amati, Giulia; Giordano, Carla; Kholová, Ivana; Preston, Stephen D.; Rizzo, Stefania; Sabatasso, Sara; et al. (2019). "Diagnosis of myocardial infarction at autopsy: AECVP reappraisal in the light of the current clinical classification
". Virchows Archiv. doi:. ISSN 0945-6317.
- This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/)
- ↑ . Chicago model for post-mortem classification of cardiomegaly. Northwestern University Feinberg School of Medicine. Retrieved on 2020-01-15.
- ↑ Kumar, Neena Theresa; Liestøl, Knut; Løberg, Else Marit; Reims, Henrik Mikael; Mæhlen, Jan (2014). "Postmortem heart weight: relation to body size and effects of cardiovascular disease and cancer ". Cardiovascular Pathology 23 (1): 5–11. doi:. ISSN 10548807.
Image sources
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 Image(s) by: Mikael Häggström, M.D. Public Domain
- Author info
- Reusing images
Autopsy of myocardial infarction
Autopsy cutting checklist
- Remove the parietal pericardium
- Separate the heart from the from lungs by cutting through the major vessels. The pulmonary artery should be cut first and the lumen inspected for any pulmonary embolism.
- Weigh the heart.
- Dissect the coronary vessels. More details in section below. Further information: Arteries
- On the right side of the heart, dissect in the direction of blood flow: Superior vena cava > right atrium > tricuspid valve > right ventricle. Look for thromboses or patent foramen ovale.[note 1]
- Dissect the atrial appendages, to exclude thromboses.
- Dissect the left ventricle, such as into circumferential slices from the apex to the base.[note 2] Inspect (and measure) the left ventricular wall thickness.
- (Measure the circumferences of the four valves. Cutoffs for valve dilatation:[1]
- Mitral valve: circumference greater than 9.9 cm in males and 9.1 cm in females
- Aortic valve: circumference greater than 8.5 cm in males and 7.9 cm in females
- Tricuspid valve: circumference greater than 11.8 cm in males and 11.1 cm in females
- Pulmonic valve: circumference greater than 7.5 cm in males and 7.4 cm in females)
Look for areas of fibrosis (Further information: Myocardial fibrosis ) or hemorrhage. Sample tissue from suspected areas, at least in cases where a diagnosis cannot be made from gross examination alone.
Gross processing of coronary arteries
Make longitudinal (or transverse cuts at 3 mm intervals[2]) through:
- The right coronary artery.
- (The right marginal artery)
- The left coronary and circumflex artery.
- The left anterior descending artery.
- (The left marginal artery)
- (The left diagonal branch)
- {{Any vessel grafts to the heart}}
Estimate the percentage of any significant stenosis or occlusion. Further information: Arteries
Plaque at different percentages of atherosclerotic stenosis.[2]
The presence of a totally occlusive thrombotic mass confers a diagnosis of likely sudden cardiac death death even in the absence of microscopically visible necrosis.[2]
Microscopy of the myocardium
By individual parameters
| Myocardial histologic parameters (HE staining)[2][3] | Earliest manifestation[2] | Full development[2] | Decrease/disappearance[2] | Image |
|---|---|---|---|---|
| Streched/wavy fibres | 0.5–4 h[3] | 
| ||
| Coagulative necrosis: cytoplasmic hypereosinophilia | 1–3 h | 1–3 days; cytoplasmic hypereosinophilia and loss of striations | > 3 days: disintegration | 
|
| Interstitial edema | 4–12 h | 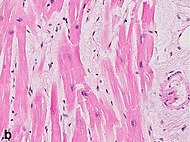
| ||
| Coagulative necrosis: ‘nuclear changes’ | 12–24 (pyknosis, karyorrhexis) | 1–3 days (loss of nuclei) | Depends on size of infarction | 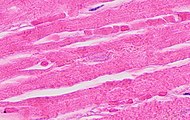
|
| Neutrophil infiltration | 12–24 h | 1–3 days | 5–7 days | 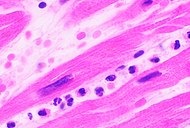
|
| Neutrophil karyorrhexis | 1.5–2 days | 3–5 days | 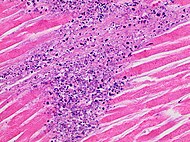
| |
| Macrophages and lymphocytes | 3–5 days | 5–10 days (including ‘siderophages’) | 10 days to 2 months | 
|
| Vessel/endothelial sprouts* | 5–10 days | 10 days–4 weeks | 4 weeks: disappearance of capillaries; some large dilated vessels persist | 
|
| Fibroblast and young collagen* | 5–10 days | 2–4 weeks | After 4 weeks; depends on size of infarction; | 
|
| Dense fibrosis Further information: Myocardial fibrosis |
4 weeks | 2–3 months | No | 
|
- Some authors summarize the vascular and early fibrotic changes as ‘granulation tissue’, which is maximal at 2–3 weeks
Chronological
| Time | Gross examination | Histopathology (light microscopy) |
|---|---|---|
| 0 - 0.5 hours | None[note 3] | None[note 3] |
| 0.5 – 4 hours | None[note 4] |
|
| 4 – 12 hours |
|
|
| 12 – 24 hours |
|
|
| 1 – 3 days |
|
|
| 3 – 7 days |
|
|
| 7 – 10 days |
|
|
| 10 – 14 days |
|
|
| 2 – 8 weeks |
|
|
| More than 2 months | Completed scarring[note 5] | Dense collagenous scar formed[note 5] |
| If not else specified in boxes, then reference is nr [5] | ||
Topography
Classify the topographic distribution of any myocardial infarction, if possible:
Reporting
Example of a normal report:
- In the myocardium, there is no evidence of fresh lesion. (The myocardium has normal texture and a homogeneous reddish brown color. No inflammation or scarring.)
See also: General notes on reporting
Notes
- ↑ The right ventricle can alternatively be cut in circumferential slices along with the left ventricle.
- ↑ An alternative approach is to cut the left ventricle through a cut along the left lateral margin, followed by an anterior cut from the apex to the aortic root, freeing the anterior wall. Then cut through the plane of the myocardium of the anterior and posterior myocardial wall, as well as the septum, for any signs of infarction. (Dissect one or more papillary muscles for infarction.)
- ↑ 3.0 3.1 For the first ~30 minutes no change at all can be seen by gross examination or by light microscopy in histopathology. However, in electron microscopy relaxed myofibrils, as well as glycogen loss and mitochondrial swelling can be observered.
- ↑ It is often possible, however, to highlight the area of necrosis that first becomes apparent after 2 to 3 hours by immersion of tissue slices in a solution of triphenyltetrazolium chloride. This dye imparts a brick-red color to intact, noninfarcted myocardium where the dehydrogenase activity is preserved. Because dehydrogenases are depleted in the area of ischemic necrosis (i.e., they leak out through the damaged cell membranes), an infarcted area is revealed as an unstained pale zone. Instead of a triphenyltetrazolium chloride dye, a LDH (lactate dehydrogenase) dye can also be used to visualize an area of necrosis.
- ↑ 5.0 5.1 Once scarring is completed, there is yet no common method of discerning the actual age of the infarct, since e.g. a scar that is four months old looks identical to a scar that is ten years old.
Main page
References
- ↑ Kitzman, Dalane W.; Scholz, David G.; Hagen, Philip T.; Ilstrup, Duane M.; Edwards, William D. (1988). "Age-Related Changes in Normal Human Hearts During the First 10 Decades of Life. Part II (Maturity): A Quantitative Anatomic Study of 765 Specimens From Subjects 20 to 99 Years Old
". Mayo Clinic Proceedings 63 (2): 137–146. doi:. ISSN 00256196.
- Griffith, Christopher C.; Raval, Jay S.; Nichols, Larry (2012). "Intravascular Talcosis due to Intravenous Drug Use Is an Underrecognized Cause of Pulmonary Hypertension
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Michaud, Katarzyna; Basso, Cristina; d’Amati, Giulia; Giordano, Carla; Kholová, Ivana; Preston, Stephen D.; Rizzo, Stefania; Sabatasso, Sara; et al. (2019). "Diagnosis of myocardial infarction at autopsy: AECVP reappraisal in the light of the current clinical classification
". Virchows Archiv. doi:. ISSN 0945-6317.
- This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/)
- ↑ 3.0 3.1 Page 547 in: Kumar, Vinay (2021). Robbins & Cotran pathologic basis of disease . Philadelphia, Pa: Elsevier. ISBN 978-0-323-60993-7. OCLC 1161987164.
- ↑ Bishop JE, Greenbaum R, Gibson DG, Yacoub M, Laurent GJ. Enhanced deposition of predominantly type I collagen in myocardial disease. J Mol Cell Cardiol. 1990;22:1157–1165
- ↑ Table 11-2 in: Mitchell, Richard Sheppard; Kumar, Vinay; Abbas, Abul K.; Fausto, Nelson. Robbins Basic Pathology . Philadelphia: Saunders. ISBN 1-4160-2973-7. 8th edition.
Image sources
Ruptured intercalated discs
Ruptured intercalated discs of myocytes of the heart have two main causes:
- Microtome processing, thereby being a visual artifact,[1] not needing reporting.
- Forceful myocardial contraction, at least in case of heart autopsy. This is mainly caused by ventricular fibrillation[2] or electric shock,[3]
If a lab often cause it as a visual artifact, it may be ignored in the report.[notes 1] If not, look for signs indicating forceful myocardial contraction, and thereby the mentioning of the findings in the report. Such signs are:[2][3]
- Alternating bundles of hypercontracted myocytes with hyperdistended ones.
- Square-shaped myocardiocyte nuclei.
- Hyperdistended myocardiocytes with detached sarcomeres, and in proximity of hypercontracted myocardiocytes.
Lung autopsy
Autopsy of the lungs, not including larger pulmonary vessels (instead summarized at Autopsy - Other thorax).
Basic autopsy cutting
In non-forensic Autopsy:
- The lungs may be cut after removing the heart through cutting through the major vessels close to it, or by removing each lung by cuts by each lung hilum.
- Dissect the pulmonary arterial system, from the pulmonary trunk and including at least segmental arteries.
- Dissect the bronchial tree, at least to segmental bronchi. Check for obstructions.
- Weigh each lung (possibly first if having cut each lung at the hilus).
- Make some additional sections through the lung parenchyma. Squeeze at each side to detect any pus and edema.[4]
- For context, see Autopsy
Gross evaluation
- A spongy consistency, and watery and frothy liquid being pressed from the parenchyma, indicates simple edema.[5]
- A spongy consistency and reddish (blood-stained) fluid being pressed from the parenchyma, indicates acute congestion.[5]
- A brownish or dark reddish color of the fluid pressed from the parenchyma indicates chronic congestion, and may not have a spongy consistency.[5]
Normal weight:
| Left | Right | |
|---|---|---|
| Men[6] | 112-675g | 155-720g |
| Women[7] | 105-515g | 101-589g |
Fixation
Generally 10% neutral buffered formalin.
See also: General notes on fixation
Microscopic evaluation
Look for the most common pathologic lung findings:[8][9]
- Alveolar fluid. Further information: Alveolar fluid
- Vascular congestion, which can usually be seen easiest in the alveolar walls. It indicates left sided heart failure, especially when seen together with alveolar fluid. Further information: Chronic pulmonary congestion
- Inflammatory cells, where a mild to moderate lymphocytic infiltrate is consistent with with heart failure, while neutrophils indicate pneumonia. pigmented macrophages of the lung may indicate chronic heart failure.
- Mycobacteria in regions of the world with substantial prevalence
- Carcinoma Further information: Lung tumor
- Aspiration: Other foreign contents in airways. Further information: Aspiration in autopsy
- Embolism of pulmonary arteries.
Carcinoma (in this case bronchioloalveolar cell adenocarcinoma) Further information: Lung tumor
Main diagnoses
- Left sided heart failure:
- Acute congestion manifests as alveolar capillaries being engorged with blood, as well as associated alveolar septal edema and/or focal intra-alveolar hemorrhage.[10]
- Chronic pulmonary congestion manifests as thickened and fibrotic septa, and alveolar spaces containing numerous pigmented lung macrophages.[10]

Additional potential findings are mentioned in the general Lungs article.
Reporting
Report findings and if they are consistent with already known diagnoses.
Example:
| Presence of sideophages indicating chronic heart failure. Prominent vessels, including alveolar capillaries, and a moderate lymphocytic infiltrate, consistent with chronic heart failure or acute decompensation. |
Further information: Autopsy
Kidney autopsy
Autopsy cutting

In non-forensic autopsy, on each side:
- After evaluating the adrenal gland, dissect the renal fascia and perirenal fat laterally, and make an incision in the renal capsule. The renal capsule can then generally be loosened by hand. Note the surface texture. (Determine the color and consistency of the kidney.)
- Dissect the kidney in the coronal plane, towards the hilum. Inspect the cut surfaces.
Gross report
The kidneys are equally sized / (of normal size, with a total weight of ___ g)((a weight of ___ g on the right side and ___ g on the right)).
| Sex | Weight, reference range[note 1] | ||
| Right kidney | Left kidney | Total | |
| Men[13] | 80–160 g (2.8–5.6 oz) | 80–175 g (2.8–6.2 oz) | 160-335g (5.6-12.8 oz) |
| Women[14] | 40–175 g (1.4–6.2 oz) | 35–190 g (1.2–6.7 oz) | 75-365g (2.6-12.9 oz) |
(No abnormal adhesions between the kidneys and surrounding fibrous capsules.)
The kidneys have smooth surfaces/ {{<<Finely / Coarsely>> granular brown surface, possibly indicating benign nephrosclerosis. There are a few cysts on the surface containing clear fluid}}. Cut surfaces have well-defined medulla, cortex, and papillae. {{The cortices and/or medullas are narrowed and congested. The papillary portions are intact.}}
The renal pelvis and ureters are unremarkable /( Renal pelvis and ureters have normal calibers, with non-irritated mucosal surfaces and open lumens).
Fixation
Generally 10% neutral buffered formalin.
See also: General notes on fixation
Microscopic evaluation

Findings
The main findings to look for:[16]
Glomerular findings
- Diffuse and/or nodular mesangial deposits:
Nodular glomerulosclerosis, prompting a consideration of diabetic nephropathy, see section below.[16]
A glomerulus with total hyalinization (also called a globally sclerotic glomerulus). People aged ≤40 should have less than 1% - 10% globally sclerotic glomeruli, and less than 30% by age 80.[17] An increased amount indicates mainly hypertensive nephropathy, see section below.[17]
- Mesangial hypercellularity (which has been defined as more than 3 cell nuclei per peripheral mesangial area on a 4-μm thick tissue section), prompting a consideration of diabetic nephropathy, see section below.[16]
- Endocapillary hypercellularity, prompting a consideration of diabetic nephropathy, see section below.[16]
- Glomerular basement membrane thickening and/or duplication, prompting a consideration of diabetic nephropathy, see section below.[16]
Proteinaceous material in Bowman's space, see Proteinaceous material in Bowman's space
- Thrombi
Tubulointerstitial findings
- Interstitial inflammation
- Cytologic atypia in the tubular epithelial cells
- Crystals
- Atypical or pigmented casts
Vascular findings
- Thrombi
- Mural deposition of amorphous material
- Vasculitis
- Congestion
Intimal thickening with an onion skin-like appearance, indicating mainly hypertensive nephropathy, see section below.
Histopathology of arteriolar hyalinosis, being a component of nephroarteriolosclerosis, also indicating mainly hypertensive nephropathy, see section below.
Diagnoses
If indicated by findings above:
Diabetic nephropathy
For alterations in glomerular matrix and/or cellularity, the most common cause is diabetic nephropathy, and typically presents as glomerular enlargement, mesangial sclerosis, basement membrane thickening and and arteriolar hyalinosis.[16]
Hypertensive nephropathy
Hypertensive nephropathy has two types:
- Benign hypertensive nephrosclerosis: Characterized by arterial or arteriolar hyalinosis, intimal fibrosis, or medial hypertrophy.[18]
- Malignant hypertensive nephrosclerosis: Characterized by fibrinoid necrosis (acute stage) or myointimal cell proliferation, usually with an “onion-skinning” appearance (chronic stage).[18]
Report
- Findings and if they are consistent with already known diagnoses.
Further information: Autopsy
Brain autopsy
Gross processing
Steps
Sample from the pituitary if it protrudes above the sella turcica. Further information: pituitary Brain:
- Weight the brain. Overall normal range (95% prediction interval) is 1100 to 1700 g,[19] +60g for males and -60g for females.[20]
- Inspect: Grooves indicating herniation? Hemorrhages?
- Dissect the basilar artery and circle of Willis, either before or after separation from the brain. [[If there is likely a need to demonstrate the case to an additional person later, the arteries of the skull base are preferably dissected after first separating them from the brain.]] Look mainly for thromboses.
- Separate the brainstem, cerebellum and cerebrum, which may be done by first separating the former two together from the cerebrum.
- Slice each part, looking for hemorrhages and/or infarcts.
- For the cerebrum, cut it into slices about 1 cm thick. It can be done from frontal to occipital, or by starting coronally into two halves at the level of the mammillary bodies and continuing in each direction from there.
- At least in people aged over 65-75 years of age {{or with suggestive history}}, look for signs of Alzhemier's disease (see picture).
Report
| The meninges and venous sinuses are unremarkable. | ((The skull is unremarkable. The calvarium is opened in the usual manner. The scalp and overlying fascia are not remarkable. The skull is <<normal in thickness {{/ somewhat thickened in the frontal areas}}. The cerebrospinal fluid is clear. The dura and venous sinuses are unremarkable. The leptomeninges are thin, shiny and non-irritated, with no visible bleeding or exudates. The superficial blood vessels are not congested. The sulci and gyri are <<normal {{/ flattened}}. |
(No visible thrombi. No epidural, subdural or subarachnoid hematoma.)
| The brain is symmetrical and weighs ___g. | ( The cerebral and cerebellar hemispheres are of equal size, and have a normal weight of ___g. [[Men: 1.180 to 1.620 g. Women: 1.030 to 1.400 g]][21][22] |
| No signs of herniation | (No grooves on the bases of the cerebrum or cerebellum.) |
((The cerebral ventricles are of normal size, with normal linings.))
Cut surfaces ((after fixation)) of the cerebrum, cerebellum and brainstem show (normal gray and white parenchyma, and) no ((encephalomalacia, ))(hemorrhages, tumors or other) focal abnormalities. ((The gyral pattern is preserved.))
The basal cerebral arteries << are ordinary / {{have mild / moderate / severe atherosclerosis}}>> without aneurysms or occlusions.
See also: General notes on reporting
Microscopy
Tissue selection
(Take samples for microscopy from:
- Cerebrum:
- Frontal lobe
- Hippocampus
- Thalamus and substantia nigra
- Cerebellum, including dentate nucleus
- Basal ganglia
- Medulla oblongata)
Look for any hemorrhage, tumor, metastatic disease, vasculitis or infarction.
Substantia nigra
In people over about 60 years of age, or in a history of suspected Parkinson's disease, look at the pars compacta for Lewy bodies and any alpha-synuclein-positive neurites, indicating Parkinson's disease.
Hippocampus
In people over about 60 years of age, or in a history of suspected Alzheimer's disease, look for its main signs in the hippocampus:
These signs are mainly visible in the temporal lobes and hippocampus proper,[23] with following images taken from its CA3 area, marked in box.
Further information: Alzheimer's disease
Basal ganglia

Look for lacunar infarcts, which are most common in the deep nuclei of the brain.[24]
Infarction
Attempt to estimate the age of any infarct. Also look at blood vessels for thrombus or stenosis.
If found, or if there is clinical suspicion of infarction in the area, look at high magnification in the pale areas for signs of infarction: The neurons become hypereosinophilic and there is an infiltrate of neutrophils. There is slight edema and loss of normal architecture in the surrounding neuropil.
Chronology at early infarction:
- At first, injured neurons shrink and become eosinophilic, with condensed nuclei. Astrocytes swell (Alzheimer type II cells).[25]
- After 6 to 8 hours, neutrophils have surrounded cerebral vessels and begin to infiltrate.[26]
A more detailed chronology is as follows:[27]
| Finding | Appearance |
|---|---|
| Eosinophilic (red) neurons | 6 hours[28] -35 days |
| Polymorphonuclear leukocytes | 1-37 days |
| Other acute neuronal injuries | 1-60 days |
| Coagulative necrosis | 1 day - 5 years |
| Spongiosis of surrounding tissue | 1 day and older |
| Astrogliosis (gemistocytes) | 2 days and older |
| Neo-vascularization | 3 days and older |
| Hemosiderin pigment | 3 days and older |
| Mononuclear inflammatory cells | 3 days–50 years |
| Macrophages | 3 days–50 years |
| Cavitation | 12 days or older |
Common normal findings
Microscopy report
Example in normal findings:
| ((Standard sections of the cerebral cortex, basal ganglia, hippocampus, thalamus/substantia nigra, medulla oblongata and cerebellum show)) intact cytoarchitecture. There is no evidence of hemorrhage, tumor, metastatic disease or vasculitis. |
Notes
- ↑ Repeated artifacts from a lab motivates measures to improve techniques.
- ↑ Renal weight range is the standard reference range, that is, defined as the interval between which 95% of values of a reference population fall into.
Main page
References
- ↑ Page 38 in: Giorgio Baroldi (2004). The Etiopathogenesis of Coronary Heart Disease: A Heretical Theory Based on Morphology, Second Edition . CRC Press. ISBN 9781498712811.
- ↑ 2.0 2.1 Page 55 in: Vittorio Fineschi, Giorgio Baroldi, Malcolm D. Silver (2016). Pathology of the Heart and Sudden Death in Forensic Medicine . CRC Press. ISBN 9781420006438.
- ↑ 3.0 3.1 Fineschi, Vittorio; Karch, Steven B.; D'Errico, Stefano; Pomara, Cristoforo; Riezzo, Irene; Turillazzi, Emanuela (2005). "Cardiac pathology in death from electrocution ". International Journal of Legal Medicine 120 (2): 79–82. doi:. ISSN 0937-9827.
- ↑ Burton, Julian L.; Rutty, Guy N. (2010). The Hospital Autopsy A Manual of Fundamental Autopsy Practice (3rd ed.). Oxford University Press. ISBN 978-0340965146.
- ↑ 5.0 5.1 5.2 page 62 in: J. Martin Beattie (2014). Post-Mortem Methods . Cambridge University Press. ISBN 9781107418004.
- ↑ Standard reference range: Molina, D. Kimberley; DiMaio, Vincent J.M. (2012). "Normal Organ Weights in Men ". The American Journal of Forensic Medicine and Pathology 33 (4): 368–372. doi:. ISSN 0195-7910.
- ↑ Standard reference range: Molina, D. Kimberley; DiMaio, Vincent J. M. (2015). "Normal Organ Weights in Women ". The American Journal of Forensic Medicine and Pathology 36 (3): 182–187. doi:. ISSN 0195-7910.
- ↑ India: Tiwana, Kanwardeep Kaur; Nibhoria, Sarita; Gupta, Manvi; Yadav, Ashish (2014). "Histopathological Spectrum in Lung Autopsies- A 50 Case Study ". Indian Journal of Forensic Medicine & Toxicology 8 (2): 172. doi:. ISSN 0973-9122.
- ↑ United States: Dr. Stanley Adams. Pulmonary Lung Conditions Found at Autopsy. Washington Forensic Services. Retrieved on 2019-12-20.
- ↑ 10.0 10.1 . Congestion. Humpath (2005-12-19).
- ↑ Madea, B (2014). Handbook of forensic medicine . Hoboken, N.J: Wiley-Blackwell. ISBN 978-1-118-57062-3. OCLC 872114659.
- ↑ Guenevere Rae, Ph.D., William Newman, M.D., Supriya Donthamsetty, M.D., Robin McGoey, M.D.. The Cadaver’s Kidney P.G.. Retrieved on 2021-05-18.
- ↑ Standard reference range: Molina, D. Kimberley; DiMaio, Vincent J.M. (2012). "Normal Organ Weights in Men ". The American Journal of Forensic Medicine and Pathology 33 (4): 368–372. doi:. ISSN 0195-7910.
- ↑ Standard reference range: Molina, D. Kimberley; DiMaio, Vincent J. M. (2015). "Normal Organ Weights in Women ". The American Journal of Forensic Medicine and Pathology 36 (3): 182–187. doi:. ISSN 0195-7910.
- ↑ Mandolin S. Ziadie, M.D.. Simple cysts. Pathology Outlines. Topic Completed: 1 November 2011. Minor changes: 1 October 2019
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 Perrone, Marie E; Chang, Anthony; Henriksen, Kammi J (2017). "Medical renal diseases are frequent but often unrecognized in adult autopsies ". Modern Pathology 31 (2): 365–373. doi:. ISSN 0893-3952.
- ↑ 17.0 17.1 Jean L. Olson. Renal Disease Caused by Hypertension. Abdominal Key. Citing:
- Kaplan C, Pasternack B, Shah H, Gallo G (1975). "Age-related incidence of sclerotic glomeruli in human kidneys. ". Am J Pathol 80 (2): 227-34. PMID 51591. PMC: 1912918. Archived from the original. .
- Kappel, Birgitte; Olsen, Steen (1980). "Cortical interstitial tissue and sclerosed glomeruli in the normal human kidney, related to age and sex ". Virchows Archiv A Pathological Anatomy and Histology 387 (3): 271–277. doi:. ISSN 0340-1227. - ↑ 18.0 18.1 Liang, Shaoshan; Le, Weibo; Liang, Dandan; Chen, Hao; Xu, Feng; Chen, Huiping; Liu, Zhihong; Zeng, Caihong (2016). "Clinico-pathological characteristics and outcomes of patients with biopsy-proven hypertensive nephrosclerosis: a retrospective cohort study ". BMC Nephrology 17 (1). doi:. ISSN 1471-2369.
- ↑ Govender, S; Lazarus, L; De-Gama, B. Z; Satyapal, K. S (2018). "Post-Mortem Brain Weight Reference Range for a Select South African Population ". International Journal of Morphology 36 (3): 915–920. doi:. ISSN 0717-9502.
- ↑ Kelley Hays; David S. (1998). Reader in Gender archaeology . Routlegde. ISBN 9780415173605. Retrieved on 2014-09-21.
- ↑ Standard reference range: Molina, D. Kimberley; DiMaio, Vincent J.M. (2012). "Normal Organ Weights in Men ". The American Journal of Forensic Medicine and Pathology 33 (4): 368–372. doi:. ISSN 0195-7910.
- ↑ Standard reference range: Molina, D. Kimberley; DiMaio, Vincent J. M. (2015). "Normal Organ Weights in Women ". The American Journal of Forensic Medicine and Pathology 36 (3): 182–187. doi:. ISSN 0195-7910.
- ↑ Rapp, Michael A.; Schnaider-Beeri, Michal; Grossman, Hillel T.; Sano, Mary; Perl, Daniel P.; Purohit, Dushyant P.; Gorman, Jack M.; Haroutunian, Vahram (2006). "Increased Hippocampal Plaques and Tangles in Patients With Alzheimer Disease With a Lifetime History of Major Depression ". Archives of General Psychiatry 63 (2): 161. doi:. ISSN 0003-990X.
- ↑ Neuropsychology : a review of science and practice, volume III . Koffler, Sandra,, Mahone, E. (E. Mark),, Marcopulos, Bernice A.,, Johnson-Greene, Douglas Eric, 1962-, Smith, Glenn E.. New York, NY. 2018-12-17. ISBN 978-0-19-065256-2. OCLC 1078637067.
- ↑ . Neuropathology, Chapter 2: Cerebral Ischemia and Stroke. Updated: October, 2017
- ↑ Jickling, Glen C; Liu, DaZhi; Ander, Bradley P; Stamova, Boryana; Zhan, Xinhua; Sharp, Frank R (2015). "Targeting Neutrophils in Ischemic Stroke: Translational Insights from Experimental Studies ". Journal of Cerebral Blood Flow & Metabolism 35 (6): 888–901. doi:. ISSN 0271-678X.
- ↑ Mărgăritescu O, Mogoantă L, Pirici I, Pirici D, Cernea D, Mărgăritescu C (2009). "Histopathological changes in acute ischemic stroke. ". Rom J Morphol Embryol 50 (3): 327-39. PMID 19690757. Archived from the original. .
- ↑ . IB. Neurons - pathological changes. Michigan State University. Retrieved on 2022-01-07.
Image sources
External link
Reproductive system
Uterus
For postmenopausal women, it is not a catastrophe if a pathology report causes the uterus to be removed as a precaution. In premenopausal women, on the other hand, any doubt regarding a pathology report that may lead to hysterectomy mandates consultation with a colleague with GYN expertise.
Cervical biopsy
Author:
Mikael Häggström [note 1]
This article includes endocervical curettage (ECC).
Comprehensiveness
On this resource, the following formatting is used for comprehensiveness:
- Minimal depth
- (Moderate depth)
- ((Comprehensive))
Fixation
Generally 10% neutral buffered formalin.
See also: General notes on fixation
Gross processing
Example report:
(Optionally: A. Container is labeled - __. The specimen is received in formalin and consists of ) 1 fragment(s) of pink-tan tissue with a vaguely recognizable mucosal surface. The tissue measures __ cm. (The surgical margin is inked black. The specimen is bisected and entirely submitted for microscopic examination in one cassette.)
Microscopic evaluationAnatomic/Histologic locationDescribe mucosa as squamous (or ectocervical), endocervical (generally mucinous and glandular) or transformation zone mucosa.
Pathologies
Further information: Cervical dysplasia
Example report
Notes
Main pageReferences
Image sources
Cervical coneUnless otherwise specified, the primary focus is any cervical neoplasia. FixationGenerally 10% neutral buffered formalin. See also: General notes on fixationGross processing
Selection and trimming
In cases where the cone is small and fragmented, try to orient the preparations and divide them if possible to obtain sagittal slices.[1] See also: General notes on gross processingMicroscopic evaluationAnatomic/Histologic locationDescribe mucosa as squamous (or ectocervical), endocervical (generally mucinous and glandular) and/or transformation zone mucosa.
Cervical dysplasia
Further information: Cervical dysplasia
RadicalityLook whether there is normal epithelium on each side of all slices where neoplasia is seen, and when the epithelium is missing in any direction, consider ordering additional serial sections or step sections. HPV changesAlso look koilocytic changes of human papillomavirus (HPV), with such cells typically displaying:
Other findings
Microscopy reportIf a neoplasia is found, the report should include:[1]
Example report in a normal case:
Endometrial polypAuthor:
Mikael Häggström [note 2] ComprehensivenessOn this resource, the following formatting is used for comprehensiveness:
FixationGenerally 10% neutral buffered formalin. See also: General notes on fixationMicroscopic evaluationThe main objectives are:
ReportingMost importantly:
Example of a minimal report:
Notes
Main pageReferences
Image sources
Endometrial curettingsGross processing
Microscopic evaluationDescribe the anatomic/histologic type of epithelium. Look mainly for:
Anatomic/histologic epithelium type(Determine the type or phase of the endometrium:)  In contrast, endocervical mucosa typically consists of mucinous columnar epithelium and mucinous glands. Evaluate this like a cervical biopsy or cervical cone. The phases of endometrium through the menstrual cycle:
If you want to specify the phase by day, then it's more accurate to state it as days past ovulation where applicable, since the follicular phase may vary substantially. Hyperplasia, atypa and/or malignancy
Microscopy reportExample in a normal case:
Endometrial thickeningHysterectomy samplingA regular hysterectomy grossing is performed, but with the following sampling:[5]
Microscopic evaluation
ReportingMost importantly:
HysterectomyFor a freshly received uterus, generally gross it fresh to include either opening it up (small specimen, no suspected malignancy seen) and/or serial sectioning, in order to let the formalin penetrate it properly. Ensure the endometrium is immersed in the formalin (such as having the serosa oriented upwards). See also: General notes on fixationComprehensivenessOn this resource, the following formatting is used for comprehensiveness:
<< Decision needed between alternatives separated by / signs >>
Gross processingGross examinationFor orientation:
When received the same day as the surgery, perform the following steps at least to serial sectioning before putting (back) in formalin, preferably with paper towels between slices, so that it fixes properly:[8]
Gross reportApplicable in bleeding disorders, pain, leiomyoma and endometrial hyperplasia:[8] Components:[8]
Slices for microscopyApplicable in bleeding disorders, pain, leiomyoma and endometrial hyperplasia:[8]
A specific sampling scheme is used in: Endometrial thickening See also: Microscopic evaluationLook for signs of malignancy:
Cervix
Further information: Cervical dysplasia
Uterine body(Determine the type or phase of the endometrium:)  In contrast, endocervical mucosa typically consists of mucinous columnar epithelium and mucinous glands. Evaluate this like a cervical biopsy or cervical cone. The phases of endometrium through the menstrual cycle:
If you want to specify the phase by day, then it's more accurate to state it as days past ovulation where applicable, since the follicular phase may vary substantially.
Microscopy reportExamples of reports:
Smooth muscle tumorGross processingGross examinationWhen finding fibroids (spherical tumors with whorled pattern, which in the uterus can be presumed to be smooth muscle tumors), examine and describe:[8]
SelectionIn case of hysterectomy, submit pieces from all fibroids >5 cm in diameter.[8] Submit any macroscopically abnormal parts of the fibroids (hemorrhagic, necrotic, brittle or softening areas, and areas with blurry delimitation).[8] For fibroids that are significantly calcified, a gross-only description as a calcified fibroid is generally sufficient, without the need to decalcify it to dissect it or sample from it.[note 3] Microscopic examinationDistinguish leiomyoma (benign) from leiomyosarcoma (malignant) by looking at the latter's criteria:[16]
Diagnosis of conventional leiomyosarcoma requires 2 of these 3 histologic features.[16]
Further information: Evaluation of tumors Microscopic reportReport:
Example, for a cervical polyp:
Intrauterine deviceGross processingThese are generally just visually described, with no samples for microscopic examination. Example report:
Products of conceptionGross processingLook up the gestational age of the pregnancy.
(If transported or processed together with other cases, put any chorionic villi in thin-mesh cassettes or tissue bags to limit contamination).[note 4] Gross report
MicroscopyThe most important is to detect the presence of chorionic villi. In the absence of chorionic villi, look for implantation site intermediate trophoblasts (ISITs):
Microscopy reportExamples:
Fallopian tubeGross processing Paratubal cysts can be ignored if incidentally found. (Look in the history for any intra-fallopian coils (Essure devices).)[note 5]
Example gross report:
Microscopic examination
TumorThe most common tumor of the fallopian tubes is adenomatoid tumor:[23]
ReportingExample of a normal report in sterilization:
When included in a uterus specimen, normal tubes and ovaries may simply be mentioned as:
 Histopathology of an ectopic pregnancy in a fallopian tube, with chorionic villi and implantation site changes Further information: Products of conception . When done for ectopic pregnancy, report any rupture, either from the gross report or from microscopy, for example:
Further information: Products of conception Notes
Main pageReferences
Image sources
OvaryGross processing "Long" and "short" axis.[1] The ovary is cut in the longitudinal plane (through the "long axis"). Ovaries, including those with cysts, are almost never inked. Gross reportTemplate:
Microscopic examination Signet ring cell carcinoma metastasis to the ovary, also called Krukenberg tumor: Gross pathology (top, cross-section at right) and histopathology at low and high magnification.[2] Apart from any obvious tumor, also look for signet ring cells, which is a major feature of metastatic tumors to the ovary.
PlacentaGross processing
 Gross pathology of placental disorders.[3] If you see a true knot (rather than "false knots" which are merely bulges or protuberances that may look like knots), report whether the diameter of the cord is significantly different before versus after the knot (which is a sign of constriction caused by the knot). Tissue selection
A membrane roll is created by cutting a strip, about 3 cm wide, of membrane, from the rupture site to the placental insertion. Hold the edge with forceps and roll it around the forceps, and then cut a transverse section of the roll.
Avoid taking placental sections near the margin. (If transported or processed together with other cases, put the placental in thin-mesh cassettes or tissue bags to limit contamination).[note 2] Example report:
Gross reportExample in a normal case:
Microscopic examination
Other relatively common findings
Microscopy reportGenerally, also include major gross findings, such as an area of placental abruption. Example of normal report:
Mild to moderate inflammation in the decidua alone can be ignored (as it is most commonly a physiological response and doesn't have a clinical significance for the fetus). Example in a twin placenta:
VulvaGross processingGenerally as per skin. Microscopic examinationMainly look for squamous vulvar intraepithelial lesions:
Staging of vulvar cancerTumors of the vulva are staged as per the AJCC, 8th Ed:[9]
Cervical cytologyClinical informationIt is not necessary to look through more than readily available reports from previous cervical cytologies. MagnificationWhile being fairly new to cervical cytology, preferably start looking at a high magnification such as 20x objective (with 10x eye piece). For suspicious findings, you may magnify up to maximum. On the other hand, once the pattern feels repetitive you can try switching to a slightly lower magnification such as 10x. AdequacyAdequacy should always be stated, either as "Satisfactory" or "Unsatisfactory". For estimating the number of cells, determine the following:
Conventional smear cellularity should be at least 8,000 cells. Liquid-based cytology cellularity should be at least 5,000 cells. Also a conventional smear is inadequate if >75% of cells are obscured by blood, exudate or air-drying artefact.[10] Eventually you will be able to tell when most cases are adequate or inadequate without performing a detailed calculation. Transformation zone presenceState whether the endocervical/transformation zone is present or absent. Count an endocervical component as present if there are 10 or more endocervical or squamous metaplastic cells.[11] In patients with previous hysterectomy, simply report glandular or squamous metaplastic cells as such, rather than stating the presence of a transformation zone, since they are likely vaginal in origin in such patients.[12] Very common findings
Main conditions to exclude or confirmSquamous atypia, seen mainly as cells with increased nucleus/cytoplasm ratio, nuclear hyperchromasia and irregular nuclear outline.
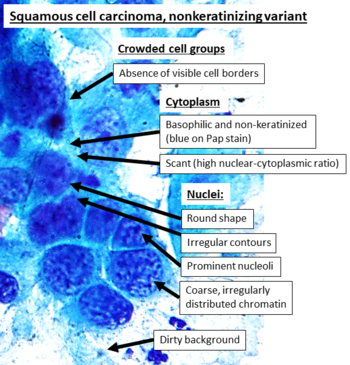 Cytopathology of squamous cell carcinoma, nonkeratinizing variant, with typical features.[14] Pap stain. Necrotic debris (dirty background) is a feature that generally makes a HSIL case "suspicious for invasive squamous cell carcinoma".[15] In contrast to the more distinct keratinizing variant, these findings are overall less specific, and most can be seen in other cancers such as adenocarcinoma as well (which, however, tends to have fine chromatin)[16] Clinical implicationIf you are uncertain of the degree of dysplasia, it can be useful to look up how much difference it will likely make for the management of the patient. You may make an Internet search for the management of abnormal cervical screening in your region (such as The ASCCP tool for management in the US). A change from close follow-up to colposcopy is not that big of a deal, but if one of the alternatives will lead to a diagnostic excision, make sure that the case is looked upon by commensurate expertise.
ReportExample in a normal case:
Male reproductive systemPhimosisGross processingGenerally sample one or two representative sections in a cassette, in addition to sections of any grossly visible lesions. See also: General notes on gross processingMicroscopic evaluationLook for:
Optionally, note any sclerosis and/or fibrosis. Reporting
Vas deferensGross processing in sterilizationIn sterilization:[19]
Communicate to the histology lab to section the specimen as tubular structures, in order to get proper cross-sections. Microscopic examination in sterilizationConfirm that there is a complete cross-section from each side. Example images of normal vas deferens: ReportExample report:
SkinTemplate:Skin - entire topic Template:Vessels, soft tissue Template:Oral and neck Template:Lungs and lymph nodes Template:Upper abdominal Template:Other surgical pathology Template:Clinical pathology - entire topic Template:Last chapters Notes
References
Image sources
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||